A multicenter evaluation of a novel microfluidic rapid AST assay for Gram-negative bloodstream infections
- PMID: 39324811
- PMCID: PMC11481479
- DOI: 10.1128/jcm.00458-24
A multicenter evaluation of a novel microfluidic rapid AST assay for Gram-negative bloodstream infections
Abstract
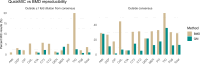
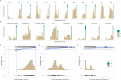
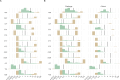
Common phenotypic methods for antimicrobial susceptibility testing (AST) of bacteria are slow, labor intensive, and display considerable technical variability. The QuickMIC system provides rapid AST using a microfluidic linear gradient. Here, we evaluate the performance of QuickMIC at four different laboratories with regard to speed, precision, accuracy, and reproducibility in comparison to broth microdilution (BMD). Spiked (n = 411) and clinical blood cultures (n = 148) were tested with the QuickMIC Gram-negative panel and compared with BMD for the 12 on-panel antibiotics, and 10 defined strains were run at each site to measure reproducibility. Logistic and linear regression analysis was applied to explore factors affecting assay performance. The overall essential agreement and categorical agreement between QuickMIC and BMD were 95.6% and 96.0%, respectively. Very major error, major error, and minor error rates were 1.0%, 0.6%, and 2.4%, respectively. Inter-laboratory reproducibility between the sites was high at 98.9% using the acceptable standard of ±1 twofold dilution. The mean in-instrument analysis time overall was 3 h 13 min (SD: 29 min). Regression analysis indicated that QuickMIC is robust with regard to initial inoculum and delay time after blood culture positivity. We conclude that QuickMIC can be used to rapidly measure MIC directly from blood cultures in clinical settings with high reproducibility, precision, and accuracy. The microfluidics-generated linear gradient ensures high reproducibility between laboratories, thus allowing a high level of trust in MIC values from single testing, at the cost of reduced measurement range compared to BMD.
Importance: Increasing antimicrobial resistance underscores the need for new diagnostic solutions to guide therapy, but traditional antimicrobial susceptibility testing (AST) is often inadequate in time-critical diseases such as sepsis. This work presents a novel and rapid AST system with a rapid turnaround of results, which may help reduce the time to guided therapy, possibly allowing early de-escalation of broad-spectrum empirical therapy as well as rapid adjustments to treatments when coverage is lacking.
Keywords: diagnostics; multicenter study; rapid AST; sepsis.
Conflict of interest statement
E.D., J.T., L.F., J.F., A.Å., J.B., H.Ö., J.Å., C.J., and C.M. are employed by Gradientech AB. T.F.-Z. was employed by Gradientech throughout the study period (2018–2022). C.M. owns stock and stock options in Gradientech. The other authors declare no conflict of interest.
Figures
References
-
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, Burden of AMR Collaborative Group . 2019. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic area in 2015: a population-level modelling analysis. Lancet Infect Dis 19:56–66. doi:10.1016/S1473-3099(18)30605-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

