Anaplasma phagocytophilum invasin AipA interacts with CD13 to elicit Src kinase signaling that promotes infection
- PMID: 39324816
- PMCID: PMC11481542
- DOI: 10.1128/mbio.01561-24
Anaplasma phagocytophilum invasin AipA interacts with CD13 to elicit Src kinase signaling that promotes infection
Abstract
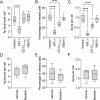
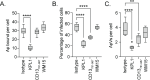
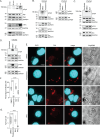
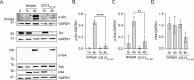
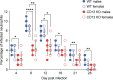
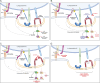
Host-microbe interactions that facilitate entry into mammalian cells are essential for obligate intracellular bacterial survival and pathogenesis. Anaplasma phagocytophilum is an obligate intracellular bacterium that invades neutrophils to cause granulocytic anaplasmosis. The invasin-receptor pairs and signaling events that induce Anaplasma uptake are inadequately defined. A. phagocytophilum invasion protein A orchestrates entry via residues 9-21 (AipA9-21) engaging an unknown receptor. Yeast two-hybrid screening suggested that AipA binds within C-terminal amino acids 851-967 of CD13 (aminopeptidase N), a multifunctional protein that, when crosslinked, initiates Src kinase and Syk signaling that culminates in endocytosis. Co-immunoprecipitation validated the interaction and confirmed that it requires the AipA N-terminus. CD13 ectopic expression on non-phagocytic cells increased susceptibility to A. phagocytophilum infection. Antibody blocking and enzymatic inhibition experiments found that the microbe exploits CD13 but not its ectopeptidase activity to infect myeloid cells. A. phagocytophilum induces Src and Syk phosphorylation during invasion. Inhibitor treatment established that Src is key for A. phagocytophilum infection, while Syk is dispensable and oriented the pathogen-invoked signaling pathway by showing that Src is activated before Syk. Disrupting the AipA-CD13 interaction with AipA9-21 or CD13781-967 antibody inhibited Src and Syk phosphorylation and also infection. CD13 crosslinking antibody that induces Src and Syk signaling restored infectivity of anti-AipA9-21-treated A. phagocytophilum. The bacterium poorly infected CD13 knockout mice, providing the first demonstration that CD13 is important for microbial infection in vivo. Overall, A. phagocytophilum AipA9-21 binds CD13 to induce Src signaling that mediates uptake into host cells, and CD13 is critical for infection in vivo.
Importance: Diverse microbes engage CD13 to infect host cells. Yet invasin-CD13 interactions, the signaling they invoke for pathogen entry, and the relevance of CD13 to infection in vivo are underexplored. Dissecting these concepts would advance fundamental understanding of a convergently evolved infection strategy and could have translational benefits. Anaplasma phagocytophilum infects neutrophils to cause granulocytic anaplasmosis, an emerging disease for which there is no vaccine and few therapeutic options. We found that A. phagocytophilum uses its surface protein and recently identified protective immunogen, AipA, to bind CD13 to elicit Src kinase signaling, which is critical for infection. We elucidated the AipA CD13 binding domain, which CD13 region AipA engages, and established that CD13 is key for A. phagocytophilum infection in vivo. Disrupting the AipA-CD13 interaction could be utilized to prevent granulocytic anaplasmosis and offers a model that could be applied to protect against multiple infectious diseases.
Keywords: Anaplasma; Anaplasmataceae; CD13; Rickettsiales; Src; Syk; bacterial internalization; host-pathogen interactions; invasin; obligate intracellular bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Binding of Host Cell Surface Protein Disulfide Isomerase by Anaplasma phagocytophilum Asp14 Enables Pathogen Infection.mBio. 2020 Jan 28;11(1):e03141-19. doi: 10.1128/mBio.03141-19. mBio. 2020. PMID: 31992623 Free PMC article.
-
Anaplasma phagocytophilum surface protein AipA mediates invasion of mammalian host cells.Cell Microbiol. 2014 Aug;16(8):1133-45. doi: 10.1111/cmi.12286. Epub 2014 Apr 3. Cell Microbiol. 2014. PMID: 24612118 Free PMC article.
-
Immunization against Anaplasma phagocytophilum Adhesin Binding Domains Confers Protection against Infection in the Mouse Model.Infect Immun. 2020 Sep 18;88(10):e00106-20. doi: 10.1128/IAI.00106-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32661123 Free PMC article.
-
Breaking in and grabbing a meal: Anaplasma phagocytophilum cellular invasion, nutrient acquisition, and promising tools for their study.Microbes Infect. 2013 Dec;15(14-15):1017-25. doi: 10.1016/j.micinf.2013.10.010. Epub 2013 Oct 18. Microbes Infect. 2013. PMID: 24141091 Free PMC article. Review.
-
Type IV secretion in the obligatory intracellular bacterium Anaplasma phagocytophilum.Cell Microbiol. 2010 Sep 1;12(9):1213-21. doi: 10.1111/j.1462-5822.2010.01500.x. Epub 2010 Jul 28. Cell Microbiol. 2010. PMID: 20670295 Free PMC article. Review.
Cited by
-
Caveolin-Mediated Endocytosis: Bacterial Pathogen Exploitation and Host-Pathogen Interaction.Cells. 2024 Dec 24;14(1):2. doi: 10.3390/cells14010002. Cells. 2024. PMID: 39791703 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
