Comparative study on the anti-inflammatory and protective effects of different oxygen therapy regimens on lipopolysaccharide-induced acute lung injury in mice
- PMID: 39324894
- PMCID: PMC11515059
- DOI: 10.4103/mgr.MEDGASRES-D-24-00044
Comparative study on the anti-inflammatory and protective effects of different oxygen therapy regimens on lipopolysaccharide-induced acute lung injury in mice
Abstract
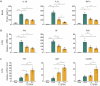
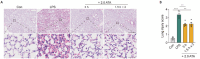
Oxygen therapy after acute lung injury can regulate the inflammatory response and reduce lung tissue injury. However, the optimal exposure pressure, duration, and frequency of oxygen therapy for acute lung injury remain unclear. In the present study, after intraperitoneal injection of lipopolysaccharide in ICR mice, 1.0 atmosphere absolute (ATA) pure oxygen and 2.0 ATA hyperbaric oxygen treatment for 1 hour decreased the levels of proinflammatory factors (interleukin-1beta and interleukin-6) in peripheral blood and lung tissues. However, only 2.0 ATA hyperbaric oxygen increased the mRNA levels of anti-inflammatory factors (interleukin-10 and arginase-1) in lung tissue; 3.0 ATA hyperbaric oxygen treatment had no significant effect. We also observed that at 2.0 ATA, the anti-inflammatory effect of a single exposure to hyperbaric oxygen for 3 hours was greater than that of a single exposure to hyperbaric oxygen for 1 hour. The protective effect of two exposures for 1.5 hours was similar to that of a single exposure for 3 hours. These results suggest that hyperbaric oxygen alleviates lipopolysaccharide-induced acute lung injury by regulating the expression of inflammatory factors in an acute lung injury model and that appropriately increasing the duration and frequency of hyperbaric oxygen exposure has a better tissue-protective effect on lipopolysaccharide-induced acute lung injury. These results could guide the development of more effective oxygen therapy regimens for acute lung injury patients.
Copyright © 2024 Copyright: © 2024 Medical Gas Research.
Conflict of interest statement
Figures





References
-
- Derwall M, Martin L, Rossaint R. The acute respiratory distress syndrome: pathophysiology, current clinical practice, and emerging therapies. Expert Rev Respir Med. 2018;12:1021–1029. - PubMed
-
- Guo H, Song Y, Li F, et al. ACT001 suppressing M1 polarization against inflammation via NF-κB and STAT1 signaling pathways alleviates acute lung injury in mice. Int Immunopharmacol. 2022;110:108944. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

