Cemiplimab monotherapy in Japanese patients with recurrent or metastatic cervical cancer
- PMID: 39325020
- PMCID: PMC11426160
- DOI: 10.1002/cam4.70236
Cemiplimab monotherapy in Japanese patients with recurrent or metastatic cervical cancer
Abstract
Background: In the phase 3 EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 study, cemiplimab significantly improved overall survival (OS) versus chemotherapy for patients with recurrent or metastatic cervical cancer who progressed after first-line platinum-based chemotherapy. We present a post hoc subgroup analysis of patients enrolled in Japan.
Methods: Patients were enrolled regardless of programmed cell death-ligand 1 status and randomized 1:1 to cemiplimab 350 mg intravenously every 3 weeks or investigator's choice single-agent chemotherapy for up to 96 weeks. Primary endpoint was OS. Key secondary endpoints were progression-free survival (PFS) and objective response rate (ORR).
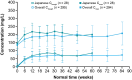
Results: Overall, 608 patients were randomized, of whom 56 (9.2%) were in Japan (cemiplimab, n = 29; chemotherapy, n = 27). The median (range) duration of follow-up was 13.6 (6.0-25.3) versus 18.2 (6.0-38.2) months for patients in Japan and for the overall population, respectively. Median OS (95% confidence interval [CI]) was 8.4 (7.0-not evaluable) and 9.4 (5.4-14.9) months for cemiplimab versus chemotherapy (hazard ratio [HR]: 0.86; 95% CI: 0.43-1.68). Median PFS (95% CI) was 4.0 (1.4-8.2) versus 3.7 (1.8-4.2) months with cemiplimab and chemotherapy (HR: 0.90; 95% CI: 0.50-1.61), respectively. ORR was 17.2% for cemiplimab and 7.4% for chemotherapy (odds ratio, 2.47; 95% CI, 0.44-13.99). Incidence of treatment-emergent adverse events at any grade was 79.3% for cemiplimab and 100% for chemotherapy. Grade ≥3 adverse events were 37.9% versus 66.7% with cemiplimab and chemotherapy, respectively.
Discussion: While acknowledging limitations inherent to a small subgroup analysis, the HR of 0.86 observed in Japanese patients suggests an emerging survival benefit despite a 4.6-month shorter median duration of follow-up versus the overall study population.
Keywords: cemiplimab; cervical cancer; chemotherapy; immunotherapy; programmed cell death‐1.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Kosei Hasegawa reports research grants from Merck Sharp & Dohme, Ono, Takeda, Daiichi‐Sankyo, and Eisai; honoraria from Takeda, Chugai Pharma, Kyowa‐Kirin, Genmab, AstraZeneca, and Merck Sharp & Dohme; and consulting/advisory board fees from Merck Sharp & Dohme, Eisai, and Takeda. Shunji Takahashi reports honoraria from Daiichi Sankyo, Eisai, Bayer, Taiho Pharmaceutical, Merck Sharp & Dohme, Novartis, Chugai Pharma, AstraZeneca, Bristol‐Myers Squibb Japan, Ono Pharmaceutical, Nihonkayaku, Pfizer, and Lilly Japan; an advisory role at Bayer; research funding from Daiichi Sankyo, Sanofi, Eisai, Bayer, Taiho Pharmaceutical, Merck Sharp & Dohme, Novartis, Chugai Pharma, AstraZeneca, Bristol‐Myers Squibb, Lilly, Ono Pharmaceutical, PharmaMar, and Pfizer/EMD Serono; and travel, accommodation, and expenses from Daiichi Sankyo and Novartis. Kimio Ushijima reports honoraria from Chugai Pharma; and research funding from Kaken Pharmaceutical, Takeda Pharmaceutical Company, and Tsumura & Co. Masao Okadome reports no conflict of interest. Kan Yonemori reports lecture fees and/or advisory fees from AstraZeneca, Chugai Pharma, Daiichi Sankyo, Esai, Ono Pharmaceutical, Novartis, Pfizer, and Takeda Pharmaceutical Company. Harushige Yokota reports honoraria from Chugai Pharma, Kaken Pharmaceutical, and Merck Sharp & Dohme; and research funding from Pfizer and Zeria Pharmaceutical. Ignace Vergote reports consulting fees from AstraZeneca, Elevar Therapeutics, Genmab, GlaxoSmithKline, Immunogen, Merck Sharp & Dohme, and Oncoinvent; and contracted research from Genmab and Hoffmann‐La Roche. Bradley J Monk reports consulting honoraria from Aravive, Asymmetric Therapeutics, Boston Biomedical, ChemoCare, ChemoID, Circulogene, Conjupro Biotherapeutics, Eisai, Geistlich, Genmab/Seattle Genetics, Gynecologic Oncology Group Foundation, ImmunoGen, Immunomedics, Incyte, Laekna Health Care, Mateon/Oxigene, Merck, Mersana, Myriad, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Precision Oncology, Puma, Regeneron, Samumed, Takeda, VBL, and Vigeo; and consulting/speaker honoraria from AstraZeneca, Clovis, Janssen/Johnson & Johnson, Roche/Genentech, and Tesaro/GSK. Krishnansu S Tewari reports honoraria from Tesaro and Clovis Oncology; consulting fees from Genentech, Tesaro, Clovis, and AstraZeneca; speaker fees from Genentech, AstraZeneca, Merck, Tesaro, and Clovis; research grants from AbbVie, Genentech, Morphotek, Merck, and Regeneron Pharmaceuticals, Inc; and travel/accommodation expenses from Genentech. Keiichi Fujiwara reports receiving consulting fees and/or grant support from Pfizer, Daiichi Sankyo, Eisai, Immunogen, Kyowa Hakko Kirin, Merck Sharp & Dohme, Mochida Pharmaceutical, NanoCarrier, Novartis, Oncotherapy, Regeneron Pharmaceuticals, Inc., Taiho, Zeria, Chugai Pharma, Genmab, and Takeda Pharmaceutical Company. Jingjin Li, Shaheda Jamil, Anne Paccaly, Frank Seebach, Israel Lowy, Melissa Mathias, and Matthew G Fury are employees and shareholders of Regeneron Pharmaceuticals, Inc. Kazuhiro Takehara reports lecture fees and consulting fees from Takeda. Tomoka Usami reports no conflict of interest. Yoichi Aoki reports no conflict of interest. Nao Suzuki reports no conflict of interest. Yoichi Kobayashi reports no conflict of interest. Yoshio Yoshida reports no conflict of interest. Hidemichi Watari reports honoraria from Merck Sharp & Dohme, Tsumura & Co, AstraZeneca, Aska Pharmaceutical Co, Ltd, Terumo, Bayer Yakuhin, Kaken Pharmaceutical, Mochida Pharmaceutical Co, Ltd, Daiichi Sankyo/UCB Japan, Chugai Pharmaceutical, and Takeda; consulting/advisory board fees from Decision Resources Group Japan, Kaken Pharmaceutical, and Chugai Pharmaceutical; and research grants from Hokkaido Welfare Federation of Agricultural Cooperatives, Kaken Pharmaceutical, Mochida Pharmaceutical Co, Ltd, Taiho Pharmaceutical, Chugai Pharmaceutical, Hokkaido Cancer Society, and Takeda. Ana Oaknin reports advisory board fees from Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Inmunogen, Genmab, Mersana Therapeutic, GlaxoSmithKline, and Deciphera Pharmaceuticals; and support for travel or accommodation from Roche, AstraZeneca, and PharmaMar.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- ICO/IARC Information Centre on HPV and Cancer . Human papillomavirus and related diseases report 2022. https://hpvcentre.net/statistics/reports/JPN.pdf
-
- Yagi A, Ueda Y, Kakuda M, et al. Epidemiologic and clinical analysis of cervical cancer using data from the population‐based Osaka cancer registry. Cancer Res. 2019;79(6):1252‐1259. - PubMed

