Arf1-dependent LRBA recruitment to Rab4 endosomes is required for endolysosome homeostasis
- PMID: 39325073
- PMCID: PMC11449124
- DOI: 10.1083/jcb.202401167
Arf1-dependent LRBA recruitment to Rab4 endosomes is required for endolysosome homeostasis
Abstract
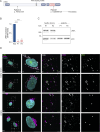
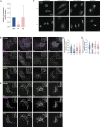
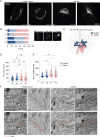
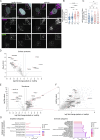
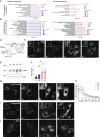
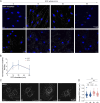
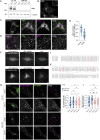
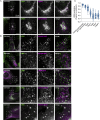
Deleterious mutations in the lipopolysaccharide responsive beige-like anchor protein (LRBA) gene cause severe childhood immune dysregulation. The complexity of the symptoms involving multiple organs and the broad range of unpredictable clinical manifestations of LRBA deficiency complicate the choice of therapeutic interventions. Although LRBA has been linked to Rab11-dependent trafficking of the immune checkpoint protein CTLA-4, its precise cellular role remains elusive. We show that LRBA, however, only slightly colocalizes with Rab11. Instead, LRBA is recruited by members of the small GTPase Arf protein family to the TGN and to Rab4+ endosomes, where it controls intracellular traffic. In patient-derived fibroblasts, loss of LRBA led to defects in the endosomal pathway promoting the accumulation of enlarged endolysosomes and lysosome secretion. Thus, LRBA appears to regulate flow through the endosomal system on Rab4+ endosomes. Our data strongly suggest functions of LRBA beyond CTLA-4 trafficking and provide a conceptual framework to develop new therapies for LRBA deficiency.
© 2024 Szentgyörgyi et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Alangari, A., Alsultan A., Adly N., Massaad M.J., Kiani I.S., Aljebreen A., Raddaoui E., Almomen A.-K., Al-Muhsen S., Geha R.S., and Alkuraya F.S.. 2012. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J. Allergy Clin. Immunol. 130:481–488.e2. 10.1016/j.jaci.2012.05.043 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

