A systematic review of dalbavancin efficacy as a sequential therapy for infective endocarditis
- PMID: 39325353
- PMCID: PMC11825564
- DOI: 10.1007/s15010-024-02393-9
A systematic review of dalbavancin efficacy as a sequential therapy for infective endocarditis
Abstract
Introduction: Dalbavancin is an antibiotic characterized by an extended half-life and efficacy against methicillin-resistant Staphylococci. Currently, there are only narrative reviews summarizing the evidence about the use of dalbavancin for infective endocarditis (IE), many of which are focused primarily on its use as consolidation therapy. For this reason, we conducted a systematic review to describe the clinical efficacy and the safety of dalbavancin in IE treatment.
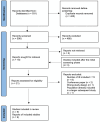
Methods: We searched for available evidence using the MEDLINE (PubMed), Embase, Scopus, Cochrane Library and Web of Science libraries, with no restrictions regarding the publication year. The risk of bias was performed using the Cochrane ROBINS-I tool for the comparative studies and the Newcastle-Ottawa Scale for descriptive studies.
Results: Nine studies were included. All of them were observational. Native valve endocarditis was the most common kind of IE found in the studies' populations (128/263, 48.7%), followed by prosthetic valve endocarditis, and cardiovascular implantable electronic device-related endocarditis. Coagulase-negative Staphylococci were the most common pathogens isolated (83/269, 30.1%), followed by S. aureus, Enterococci spp and Streptococci spp. Five out of nine studies documented a clinical failure rate of less than 10%. Dalbavancin showed a favourable safety profile. Dalbavancin appears to be a promising option for the consolidation therapy of IE. However, further studies comparing dalbavancin with standard of care are needed.
Prospero registration number: CRD42023430032.
Keywords: Dalbavancin; Gram-positive; Infective endocarditis; Long acting antibiotics; Sequential therapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: JPH has participated in educational programs organized by MSD, Pfizer, Menarini and Angelini, and in advisory boards organized by Advanz Pharma, Tillots, and GILEAD. The other authors declare no conflict of interest.
References
-
- Momtazmanesh S, Saeedi Moghaddam S, Malakan Rad E, Azadnajafabad S, Ebrahimi N, Mohammadi E, et al. Global, regional, and national burden and quality of care index of endocarditis: the global burden of disease study 1990–2019. Eur J Prev Cardiol. 2022;29:1287–97. 10.1093/eurjpc/zwab211. - PubMed
-
- Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222–32. 10.1093/eurheartj/ehz620. - PubMed
-
- Sader HS, Mendes RE, Pfaller MA, Flamm RK. Antimicrobial activity of dalbavancin tested against Gram-positive organisms isolated from patients with infective endocarditis in US and European medical centres. J Antimicrob Chemother. 2019;74:1306–10. 10.1093/jac/dkz006. - PubMed
-
- Lefort A, Pavie J, Garry L, Chau F, Fantin B. Activities of Dalbavancin in Vitro and in a rabbit model of experimental endocarditis due to Staphylococcus aureus with or without reduced susceptibility to Vancomycin and Teicoplanin. Antimicrob Agents Chemother. 2004;48:1061–4. 10.1128/AAC.48.3.1061-1064.2004. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous