Oral Microbiome and Subsequent Risk of Head and Neck Squamous Cell Cancer
- PMID: 39325441
- PMCID: PMC11428028
- DOI: 10.1001/jamaoncol.2024.4006
Oral Microbiome and Subsequent Risk of Head and Neck Squamous Cell Cancer
Abstract
Importance: The oral microbiota may be involved in development of head and neck squamous cell cancer (HNSCC), yet current evidence is largely limited to bacterial 16S amplicon sequencing or small retrospective case-control studies.
Objective: To test whether oral bacterial and fungal microbiomes are associated with subsequent risk of HNSCC development.
Design, setting, and participants: Prospective nested case-control study among participants providing oral samples in 3 epidemiological cohorts, the American Cancer Society Cancer Prevention Study II Nutrition Cohort, the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, and the Southern Community Cohort Study. Two hundred thirty-six patients who prospectively developed HNSCC were identified during a mean (SD) of 5.1 (3.6) years of follow-up. Control participants who remained HNSCC free were selected by 2:1 frequency matching on cohort, age, sex, race and ethnicity, and time since oral sample collection. Data analysis was conducted in 2023.
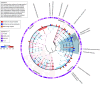
Exposures: Characterization of the oral bacterial microbiome using whole-genome shotgun sequencing and the oral fungal microbiome using internal transcribed spacer sequencing. Association of bacterial and fungal taxa with HNSCC was assessed by analysis of compositions of microbiomes with bias correction. Association with red and orange oral pathogen complexes was tested by logistic regression. A microbial risk score for HNSCC risk was calculated from risk-associated microbiota.
Main outcomes and measures: The primary outcome was HNSCC incidence.
Results: The study included 236 HNSCC case participants with a mean (SD) age of 60.9 (9.5) years and 24.6% women during a mean of 5.1 (3.6) years of follow-up, and 485 matched control participants. Overall microbiome diversity at baseline was not related to subsequent HNSCC risk; however 13 oral bacterial species were found to be differentially associated with development of HNSCC. The species included the newly identified Prevotella salivae, Streptococcus sanguinis, and Leptotrichia species, as well as several species belonging to beta and gamma Proteobacteria. The red/orange periodontal pathogen complex was moderately associated with HNSCC risk (odds ratio, 1.06 per 1 SD; 95% CI, 1.00-1.12). A 1-SD increase in microbial risk score (created based on 22 bacteria) was associated with a 50% increase in HNSCC risk (multivariate odds ratio, 1.50; 95% CI, 1.21-1.85). No fungal taxa associated with HNSCC risk were identified.
Conclusions and relevance: This case-control study yielded compelling evidence that oral bacteria are a risk factor for HNSCC development. The identified bacteria and bacterial complexes hold promise, along with other risk factors, to identify high-risk individuals for personalized prevention of HNSCC.
Conflict of interest statement
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

