A Digital Cognitive Behavioral Therapy Program for Adults With Alcohol Use Disorder: A Randomized Clinical Trial
- PMID: 39325452
- PMCID: PMC11428014
- DOI: 10.1001/jamanetworkopen.2024.35205
A Digital Cognitive Behavioral Therapy Program for Adults With Alcohol Use Disorder: A Randomized Clinical Trial
Abstract
Importance: Cognitive behavioral therapy (CBT) is an evidence-based treatment for alcohol use, yet patient access is limited and may be enhanced through digital therapeutics.
Objective: To evaluate the efficacy of a digital CBT program (Computer-Based Training for Cognitive Behavioral Therapy [CBT4CBT]) or clinician-delivered CBT compared with standard treatment for reducing alcohol use.
Design, setting, and participants: A 3-arm randomized clinical trial was conducted at outpatient substance use treatment facilities in Connecticut between February 14, 2017, and December 31, 2021, that included an 8-week treatment period with a 6-month follow-up period. Treatment-seeking adults were included who met criteria for current alcohol use disorder and reported drinking at least 14 (men) or 7 (women) drinks per week in the past month and were sufficiently stable for outpatient treatment.
Interventions: Participants were randomly assigned to 1 of the following groups: (1) treatment as usual (TAU) consisting of weekly group or individual counseling, (2) CBT delivered weekly by trained and fidelity-monitored clinicians, or (3) web-based CBT plus brief weekly clinical monitoring.
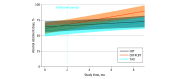
Main outcomes and measures: Rates of alcohol use were measured weekly during the treatment period and at 1-, 3-, and 6-month follow-up using the timeline follow-back method. The primary outcome was the percentage of days abstinent (PDA) from alcohol per month. Intention-to-treat analyses were conducted.
Results: Of the 99 randomized participants (mean [SD] age, 45.5 [12.7]), 66 were male (66.7%); 39 identified as Black/African American (39.8%), 19 (19.2%) as Hispanic, and 47 (48.0%) as White. Mean (SD) rates of PDA from baseline to 6-month follow-up were 49.3% (27.8%) to 69.6% (34.4%) for TAU; 53.7% (29.8%) to 70.2% (35.1%) for CBT; and 47.6% (31.8%) to 82.6% (25.3%) for digital CBT. Results of random-effects regression showed a significant increase in PDA during the study period, with those assigned to digital CBT increasing PDA at a faster rate than TAU (t733 = 2.55; P = .01) and CBT (t733 = 3.36; P < .001). However, there was no statistically significant difference between treatment groups during the 8-week treatment period.
Conclusions and relevance: In this randomized clinical trial, while there was no significant difference between treatment groups during the 8-week treatment period, there was differential change between treatments during the 8-month study period that provides support for the efficacy of this digital CBT program.
Trial registration: ClinicalTrials.gov Identifier: NCT02742246.
Conflict of interest statement
Figures
Comment in
-
Digital Cognitive Behavioral Therapy Intervention for Alcohol Use Disorder.JAMA Netw Open. 2024 Sep 3;7(9):e2435216. doi: 10.1001/jamanetworkopen.2024.35216. JAMA Netw Open. 2024. PMID: 39325458 No abstract available.
References
-
- American Psychiatric Association . Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

