Immunomodulatory Therapy for Ischemic Heart Disease
- PMID: 39325497
- PMCID: PMC11521113
- DOI: 10.1161/CIRCULATIONAHA.124.070368
Immunomodulatory Therapy for Ischemic Heart Disease
Abstract
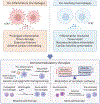
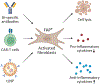
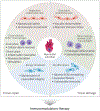
Ischemic heart disease is a leading cause of death worldwide, manifested clinically as myocardial infarction (and ischemic cardiomyopathy. Presently, there exists a notable scarcity of efficient interventions to restore cardiac function after myocardial infarction. Cumulative evidence suggests that impaired tissue immunity within the ischemic microenvironment aggravates cardiac dysfunction, contributing to progressive heart failure. Recent research breakthroughs propose immunotherapy as a potential approach by leveraging immune and stroma cells to recalibrate the immune microenvironment, holding significant promise for the treatment of ischemic heart disease. In this Primer, we highlight three emerging strategies for immunomodulatory therapy in managing ischemic cardiomyopathy: targeting vascular endothelial cells to rewire tissue immunity, reprogramming myeloid cells to bolster their reparative function, and utilizing adoptive T cell therapy to ameliorate fibrosis. We anticipate that immunomodulatory therapy will offer exciting opportunities for ischemic heart disease treatment.
Keywords: chimeric antigen receptor; endothelial cells; fibroblasts; fibrosis; immunotherapy; ischemic heart disease; macrophages; myeloid cells; myocardial infarction.
Conflict of interest statement
J.A.E. is a scientific founder and holds equity in Capstan Therapeutics, which develops therapeutics to reprogram immune cells in vivo. The other authors report no conflicts.
Figures

References
-
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah P, Willerson JT, Benza RL, Berman DS. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

