YTHDF1 loss in dendritic cells potentiates radiation-induced antitumor immunity via STING-dependent type I IFN production
- PMID: 39325547
- PMCID: PMC11601937
- DOI: 10.1172/JCI181612
YTHDF1 loss in dendritic cells potentiates radiation-induced antitumor immunity via STING-dependent type I IFN production
Abstract
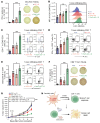
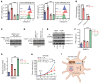
The RNA N6-methyladenosine (m6A) reader YTHDF1 is implicated in cancer etiology and progression. We discovered that radiotherapy (RT) increased YTHDF1 expression in dendritic cells (DCs) of PBMCs from patients with cancer, but not in other immune cells tested. Elevated YTHDF1 expression in DCs was associated with poor outcomes for patients receiving RT. We found that loss of Ythdf1 in DCs enhanced the antitumor effects of ionizing radiation (IR) by increasing the cross-priming capacity of DCs across multiple murine cancer models. Mechanistically, IR upregulated YTHDF1 expression in DCs through stimulator of IFN genes/type I IFN (STING/IFN-I) signaling. YTHDF1 in turn triggered STING degradation by increasing lysosomal cathepsins, thereby reducing IFN-I production. We created a YTHDF1 deletion/inhibition prototype DC vaccine that significantly improved the therapeutic effect of RT and radioimmunotherapy in a murine melanoma model. Our findings reveal a layer of regulation between YTHDF1/m6A and STING in response to IR, which opens new paths for the development of YTHDF1-targeting therapies.
Keywords: Cancer immunotherapy; Dendritic cells; Immunology; Oncology; Radiation therapy.
Figures




Comment in
- Taking the STING out of radiotherapy: STING checkpoints mediate radiation resistance
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

