The human milk oligosaccharide 3'sialyllactose reduces low-grade inflammation and atherosclerosis development in mice
- PMID: 39325548
- PMCID: PMC11601559
- DOI: 10.1172/jci.insight.181329
The human milk oligosaccharide 3'sialyllactose reduces low-grade inflammation and atherosclerosis development in mice
Abstract
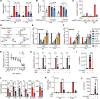
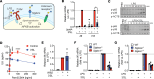
Macrophages contribute to the induction and resolution of inflammation and play a central role in chronic low-grade inflammation in cardiovascular diseases caused by atherosclerosis. Human milk oligosaccharides (HMOs) are complex unconjugated glycans unique to human milk that benefit infant health and act as innate immune modulators. Here, we identify the HMO 3'sialyllactose (3'SL) as a natural inhibitor of TLR4-induced low-grade inflammation in macrophages and endothelium. Transcriptome analysis in macrophages revealed that 3'SL attenuates mRNA levels of a selected set of inflammatory genes and promotes the activity of liver X receptor (LXR) and sterol regulatory element binding protein-1 (SREBP1). These acute antiinflammatory effects of 3'SL were associated with reduced histone H3K27 acetylation at a subset of LPS-inducible enhancers distinguished by preferential enrichment for CCCTC-binding factor (CTCF), IFN regulatory factor 2 (IRF2), B cell lymphoma 6 (BCL6), and other transcription factor recognition motifs. In a murine atherosclerosis model, both s.c. and oral administration of 3'SL significantly reduced atherosclerosis development and the associated inflammation. This study provides evidence that 3'SL attenuates inflammation by a transcriptional mechanism to reduce atherosclerosis development in the context of cardiovascular disease.
Keywords: Atherosclerosis; Cell biology; Epigenetics; Inflammation; Macrophages.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

