Maternal Respiratory Syncytial Virus Vaccination and Receipt of Respiratory Syncytial Virus Antibody (Nirsevimab) by Infants Aged <8 Months - United States, April 2024
- PMID: 39325675
- PMCID: PMC11563570
- DOI: 10.15585/mmwr.mm7338a2
Maternal Respiratory Syncytial Virus Vaccination and Receipt of Respiratory Syncytial Virus Antibody (Nirsevimab) by Infants Aged <8 Months - United States, April 2024
Abstract
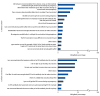
Respiratory syncytial virus (RSV) is the most common cause of hospitalization among U.S. infants. CDC recommends RSV vaccination for pregnant persons or administration of RSV antibody (nirsevimab) to infants aged <8 months to prevent RSV lower respiratory tract disease among infants. To determine maternal and infant RSV immunization coverage for the 2023-24 RSV season, CDC conducted an Internet panel survey during March 26-April 11, 2024. Among 678 women at 32-36 weeks' gestation during September 2023-January 2024, 32.6% reported receipt of an RSV vaccine any time during pregnancy. Among 866 women with an infant born during August 2023-March 2024, 44.6% reported receipt of nirsevimab by the infant. Overall, 55.8% of infants were protected by maternal RSV vaccine, nirsevimab, or both. Provider recommendation for maternal vaccination or infant nirsevimab was associated with higher immunization coverage, whereas lack of a provider recommendation was the main reason for not getting RSV immunization. The main reason for definitely or probably not getting nirsevimab for infants was concern about the long-term safety for the infant. Activities supporting providers to make RSV prevention recommendations and have informative conversations with patients might increase the proportion of infants protected against severe RSV disease. CDC and the American College of Obstetricians and Gynecologists have resources to assist providers in effectively communicating the importance of immunization.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–22. 10.15585/mmwr.mm7241e1 - DOI - PMC - PubMed
-
- Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:920–5. 10.15585/mmwr.mm7234a4 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

