PPARγ agonist alleviates calcium oxalate nephrolithiasis by regulating mitochondrial dynamics in renal tubular epithelial cell
- PMID: 39325731
- PMCID: PMC11426502
- DOI: 10.1371/journal.pone.0310947
PPARγ agonist alleviates calcium oxalate nephrolithiasis by regulating mitochondrial dynamics in renal tubular epithelial cell
Abstract
Background: Kidney stone formation is a common disease that causes a significant threat to human health. The crystallization mechanism of calcium oxalate, the most common type of kidney stone, has been extensively researched, yet the damaging effects and mechanisms of calcium oxalate crystals on renal tubular epithelial cells remain incompletely elucidated. Regulated mitochondrial dynamics is essential for eukaryotic cells, but its role in the occurrence and progression of calcium oxalate (CaOx) nephrolithiasis is not yet understood.
Methods: An animal model of calcium oxalate-related nephrolithiasis was established in adult male Sprague‒Dawley (SD) rats by continuously administering drinking water containing 1% ethylene glycol for 28 days. The impact of calcium oxalate crystals on mitochondrial dynamics and apoptosis in renal tubular epithelial cells was investigated using HK2 cells in vitro. Blood samples and bilateral kidney tissues were collected for histopathological evaluation and processed for tissue injury, inflammation, fibrosis, oxidative stress detection, and mitochondrial dynamics parameter analysis.
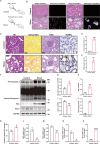
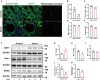
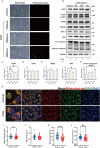
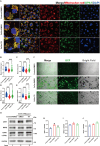
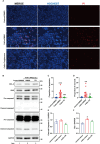
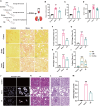
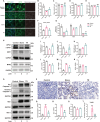
Results: Calcium oxalate crystals caused higher levels of mitochondrial fission and apoptosis in renal tubular epithelial cells both in vivo and in vitro. Administration of a PPARγ agonist significantly alleviated mitochondrial fission and apoptosis in renal tubular epithelial cells, and improved renal function, accompanied by reduced levels of oxidative stress, increased antioxidant enzyme expression, alleviation of inflammation, and reduced fibrosis in vivo.
Conclusion: Our results indicated that increased mitochondrial fission in renal tubular epithelial cells is a critical component of kidney injury caused by calcium oxalate stones, leading to the accumulation of reactive oxygen species within the tissue and the subsequent initiation of apoptosis. Regulating mitochondrial dynamics represents a promising approach for calcium oxalate nephrolithiasis.
Copyright: © 2024 Liu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Mitochondrial enzyme HIBADH protects against calcium oxalate nephrolithiasis by modulating oxidative stress and apoptosis.Arch Biochem Biophys. 2025 Aug;770:110452. doi: 10.1016/j.abb.2025.110452. Epub 2025 May 5. Arch Biochem Biophys. 2025. PMID: 40334962
-
Rosiglitazone Suppresses Calcium Oxalate Crystal Binding and Oxalate-Induced Oxidative Stress in Renal Epithelial Cells by Promoting PPAR-γ Activation and Subsequent Regulation of TGF-β1 and HGF Expression.Oxid Med Cell Longev. 2019 Nov 12;2019:4826525. doi: 10.1155/2019/4826525. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31781338 Free PMC article.
-
Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney.Redox Biol. 2018 Jun;16:414-425. doi: 10.1016/j.redox.2018.03.019. Epub 2018 Apr 2. Redox Biol. 2018. PMID: 29653411 Free PMC article.
-
Nephrolithiasis: a consequence of renal epithelial cell exposure to oxalate and calcium oxalate crystals.Mol Urol. 2000 Winter;4(4):305-12. Mol Urol. 2000. PMID: 11156696 Review.
-
Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development.Urol Res. 1995;23(2):71-9. doi: 10.1007/BF00307936. Urol Res. 1995. PMID: 7676537 Review.
Cited by
-
Apoptosis, ferroptosis, necrosis, necroptosis and pyroptosis in the formation of calcium oxalate kidney stones.Urolithiasis. 2025 Aug 11;53(1):153. doi: 10.1007/s00240-025-01826-w. Urolithiasis. 2025. PMID: 40788511 Review.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

