Lawsonia intracellularis regulates nuclear factor-κB signalling pathway during infection
- PMID: 39325775
- PMCID: PMC11426430
- DOI: 10.1371/journal.pone.0310804
Lawsonia intracellularis regulates nuclear factor-κB signalling pathway during infection
Abstract
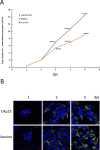
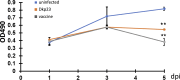
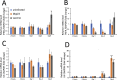
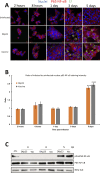
Lawsonia intracellularis is the etiological agent of proliferative enteropathy (PE) in pigs, horses and wide range of mammals. Little is known about the role of innate immune response during L. intracellularis infection. In this study, we investigated the nuclear factor-κB (NF-κB)-regulated immune response against infection of a clinical strain Dkp23 and a live-attenuated Enterisol vaccine strain in PK-15 cells. We found that expression of NF-κB target genes TNF-α, IFN-γ, IL-6 and IL-8 were modulated during the course of infection. At 5 dpi, there was a significant increase in p65 NF-κB activation, including protein nuclear translocation and phosphorylation, synchronous with the induction of IL-6, IFN-γ and IL-8 expression in L. intracellularis infected cells, especially for Enterisol vaccine strain-infected cells. This result suggests that NF-κB signalling level is induced when L. intracellularis bacterial load peaks at 5 dpi. The induction of pro-inflammatory cytokines expression is consistent with the decreased viability of L. intracellularis-infected cells especially that of the vaccine strain. There were no significant changes in NF-κB signalling between vaccine and Dkp23 infection in PK-15 cells, except for moderate levels of differences in NF-κB target genes expression which might be a reflection of differences in intracellular bacterial load. Overall, the data presented here indicate a correlation between the induction of NF-κB signalling and the L. intracellularis bacterial load in PK-15 cells.
Copyright: © 2024 Yang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Systemic cytokine response in pigs infected orally with a Lawsonia intracellularis isolate of South Korean origin.J Vet Med Sci. 2018 Jan 1;80(1):13-19. doi: 10.1292/jvms.17-0036. Epub 2017 Nov 15. J Vet Med Sci. 2018. PMID: 29142159 Free PMC article.
-
Vaccination of pigs with attenuated Lawsonia intracellularis induced acute phase protein responses and primed cell-mediated immunity without reduction in bacterial shedding after challenge.Vaccine. 2015 Jan 1;33(1):156-62. doi: 10.1016/j.vaccine.2014.10.084. Epub 2014 Nov 11. Vaccine. 2015. PMID: 25444804
-
Evidence of cell-mediated immune response and specific local mucosal immunoglobulin (Ig) A production against Lawsonia intracellularis in experimentally infected swine.Can J Vet Res. 2010 Apr;74(2):97-101. Can J Vet Res. 2010. PMID: 20592838 Free PMC article.
-
Recent advances in understanding the pathogenesis of Lawsonia intracellularis infections.Vet Pathol. 2014 Mar;51(2):465-77. doi: 10.1177/0300985813520249. Epub 2014 Jan 29. Vet Pathol. 2014. PMID: 24476941 Review.
-
Immune response and protection against Lawsonia intracellularis infections in pigs.Vet Immunol Immunopathol. 2020 Jan;219:109959. doi: 10.1016/j.vetimm.2019.109959. Epub 2019 Oct 31. Vet Immunol Immunopathol. 2020. PMID: 31710909 Review.
References
-
- Cooper DM, Swanson DL, Gebhart CJ. Diagnosis of proliferative enteritis in frozen and formalin-fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using a Lawsonia intracellularis-specific multiplex PCR assay. Vet Microbiol. 1997;54(1):47–62. Epub 1997/01/01. doi: 10.1016/s0378-1135(96)01264-3 . - DOI - PubMed
-
- Kroll JJ, Eichmeyer MA, Schaeffer ML, McOrist S, Harris DL, Roof MB. Lipopolysaccharide-based enzyme-linked immunosorbent assay for experimental use in detection of antibodies to Lawsonia intracellularis in pigs. Clin Diagn Lab Immunol. 2005;12(6):693–9. Epub 2005/06/09. doi: 10.1128/CDLI.12.6.693-699.2005 ; PubMed Central PMCID: PMC1151981. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

