Production and characterization of homologous protoporphyrinogen IX oxidase (PPO) proteins: Evidence that small N-terminal amino acid changes do not impact protein function
- PMID: 39325813
- PMCID: PMC11426539
- DOI: 10.1371/journal.pone.0311049
Production and characterization of homologous protoporphyrinogen IX oxidase (PPO) proteins: Evidence that small N-terminal amino acid changes do not impact protein function
Abstract
Transgenic soybean, cotton, and maize tolerant to protoporphyrinogen IX oxidase (PPO)-inhibiting herbicides have been developed by introduction of a bacterial-derived PPO targeted into the chloroplast. PPO is a membrane-associated protein with an intrinsic tendency for aggregation, making expression, purification, and formulation at high concentrations difficult. In this study, transgenic PPO expressed in three crops was demonstrated to exhibit up to a 13 amino acid sequence difference in the N-terminus due to differential processing of the chloroplast transit peptide (CTP). Five PPO protein variants were produced in and purified from E. coli, each displaying equivalent immunoreactivity and functional activity, with values ranging from 193 to 266 nmol min-1 mg-1. Inclusion of an N-terminal 6xHis-tag or differential processing of the CTP peptide does not impact PPO functional activity. Additionally, structural modeling by Alphafold, ESMfold, and Openfold indicates that these short N-terminal extensions are disordered and predicted to not interfere with the mature PPO structure. These results support the view that safety studies on PPO from various crops can be performed from a single representative variant. Herein, we report a novel and robust method for large-scale production of PPO, enabling rapid production of more than 200 g of highly active PPO protein at 99% purity and low endotoxin contamination. We also present a formulation that allows for concentration of active PPO to > 75 mg/mL in a buffer suitable for mammalian toxicity studies.
Copyright: © 2024 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

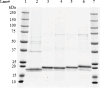
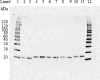

Similar articles
-
Risk assessment of homologous variants of biotech trait proteins using a bridging approach.GM Crops Food. 2024 Dec 31;15(1):336-351. doi: 10.1080/21645698.2024.2420412. Epub 2024 Nov 9. GM Crops Food. 2024. PMID: 39520709 Free PMC article.
-
Identification of Escherichia coli HemG as a novel, menadione-dependent flavodoxin with protoporphyrinogen oxidase activity.Biochemistry. 2009 Jul 28;48(29):6705-11. doi: 10.1021/bi900850y. Biochemistry. 2009. PMID: 19583219 Free PMC article.
-
Recombinant maize protoporphyrinogen IX oxidase expressed in Escherichia coli forms complexes with GroEL and DnaK chaperones.Protein Expr Purif. 2000 Oct;20(1):81-6. doi: 10.1006/prep.2000.1274. Protein Expr Purif. 2000. PMID: 11035954
-
Protoporphyrinogen oxidase inhibitor: an ideal target for herbicide discovery.Chimia (Aarau). 2011;65(12):961-9. doi: 10.2533/chimia.2011.961. Chimia (Aarau). 2011. PMID: 22273380 Review.
-
Development of PPO inhibitor-resistant cultures and crops.Pest Manag Sci. 2005 Mar;61(3):277-85. doi: 10.1002/ps.1011. Pest Manag Sci. 2005. PMID: 15660355 Review.
References
-
- Singh M, Jhala AJ, Kukal MS, Irmak S. Water use characteristics of weeds: a global review, best practices, and future directions. Frontiers in Plant Science. 2022;13(January). doi: 10.3389/fpls.2021.794090 https://www.frontiersin.org/articles/10.3389/fpls.2021.794090/full. 20220271046. Singh, M. (author). - DOI - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

