The I7L protein of African swine fever virus is involved in viral pathogenicity by antagonizing the IFN-γ-triggered JAK-STAT signaling pathway through inhibiting the phosphorylation of STAT1
- PMID: 39325821
- PMCID: PMC11460700
- DOI: 10.1371/journal.ppat.1012576
The I7L protein of African swine fever virus is involved in viral pathogenicity by antagonizing the IFN-γ-triggered JAK-STAT signaling pathway through inhibiting the phosphorylation of STAT1
Abstract
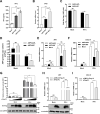
Cell-passage-adapted strains of African swine fever virus (ASFV) typically exhibit substantial genomic alterations and attenuated virulence in pigs. We have indicated that the human embryonic kidney (HEK293T) cells-adapted ASFV strain underwent genetic alterations and the I7L gene in the right variable region was deleted compared with the ASFV HLJ/2018 strain (ASFV-WT). A recent study has revealed that the deletion of the I7L-I11L genes results in attenuation of virulent ASFV in vivo, but the underlying mechanism remains largely unknown. Therefore, we hypothesized that the deletion of the I7L gene may be related to the pathogenicity of ASFV in pigs. We generated the I7L gene-deleted ASFV mutant (ASFV-ΔI7L) and found that the I7L gene deletion does not influence the replication of ASFV in primary porcine alveolar macrophages (PAMs). Using transcriptome sequencing analysis, we identified that the differentially expressed genes in the PAMs infected with ASFV-ΔI7L were mainly involved in antiviral immune responses induced by interferon gamma (IFN-γ) compared with those in the ASFV-WT-infected PAMs. Meanwhile, we further confirmed that the I7L protein (pI7L) suppressed the IFN-γ-triggered JAK-STAT signaling pathway. Mechanistically, pI7L interacts with STAT1 and inhibits its phosphorylation and homodimerization, which depends on the tyrosine at position 98 (Y98) of pI7L, thereby preventing the nuclear translocation of STAT1 and leading to the decreased production of IFN-γ-stimulated genes. Importantly, ASFV-ΔI7L exhibited reduced replication and virulence compared with ASFV-WT in pigs, likely due to the increased production of IFN-γ-stimulated genes, indicating that pI7L is involved in the virulence of ASFV. Taken together, our findings demonstrate that pI7L is associated with pathogenicity and antagonizes the IFN-γ-triggered JAK-STAT signaling pathway via inhibiting the phosphorylation and homodimerization of STAT1 depending on the Y98 residue of pI7L and the Src homology 2 domain of STAT1, which provides more information for understanding the immunoevasion strategies and designing the live attenuated vaccines against ASFV infection.
Copyright: © 2024 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
MGF360-9L Is a Major Virulence Factor Associated with the African Swine Fever Virus by Antagonizing the JAK/STAT Signaling Pathway.mBio. 2022 Feb 22;13(1):e0233021. doi: 10.1128/mbio.02330-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35076286 Free PMC article.
-
The A137R Protein of African Swine Fever Virus Inhibits Type I Interferon Production via the Autophagy-Mediated Lysosomal Degradation of TBK1.J Virol. 2022 May 11;96(9):e0195721. doi: 10.1128/jvi.01957-21. Epub 2022 Apr 12. J Virol. 2022. PMID: 35412346 Free PMC article.
-
African Swine Fever Virus E120R Protein Inhibits Interferon Beta Production by Interacting with IRF3 To Block Its Activation.J Virol. 2021 Aug 25;95(18):e0082421. doi: 10.1128/JVI.00824-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190598 Free PMC article.
-
African swine fever viral proteins that inhibit cGAS-STING pathway and type-I interferon production.Virology. 2025 Jan;602:110317. doi: 10.1016/j.virol.2024.110317. Epub 2024 Nov 26. Virology. 2025. PMID: 39616703 Review.
-
Identification and utility of innate immune system evasion mechanisms of ASFV.Virus Res. 2013 Apr;173(1):87-100. doi: 10.1016/j.virusres.2012.10.013. Epub 2012 Nov 16. Virus Res. 2013. PMID: 23165138 Review.
Cited by
-
Advances in African swine fever virus molecular biology and host interactions contributing to new tools for control.J Virol. 2025 Jun 17;99(6):e0093224. doi: 10.1128/jvi.00932-24. Epub 2025 May 9. J Virol. 2025. PMID: 40340396 Free PMC article. Review.
-
Immunoevasion strategies for African swine fever virus: Modulation of antigen presentation pathways.Virulence. 2025 Dec;16(1):2541711. doi: 10.1080/21505594.2025.2541711. Epub 2025 Aug 15. Virulence. 2025. PMID: 40817451 Free PMC article. Review.
-
Resveratrol inhibits African swine fever virus replication via the Nrf2-mediated reduced glutathione and antioxidative activities.Emerg Microbes Infect. 2025 Dec;14(1):2469662. doi: 10.1080/22221751.2025.2469662. Epub 2025 Mar 3. Emerg Microbes Infect. 2025. PMID: 39964001 Free PMC article.
-
African swine fever virus MGF505-4R facilitates cGAS degradation through TOLLIP-mediated selective autophagy and inhibits the formation of ISGF3 to evade innate immunity.Vet Res. 2025 Jul 5;56(1):137. doi: 10.1186/s13567-025-01569-x. Vet Res. 2025. PMID: 40618140 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

