Singlet Oxygen Produced by Aspalathin and Ascorbic Acid Leads to Fragmentation of Dihydrochalcones and Adduct Formation
- PMID: 39326013
- PMCID: PMC11468778
- DOI: 10.1021/acs.jafc.4c07633
Singlet Oxygen Produced by Aspalathin and Ascorbic Acid Leads to Fragmentation of Dihydrochalcones and Adduct Formation
Abstract
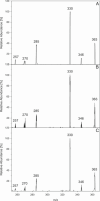
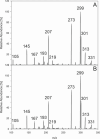
Singlet oxygen-mediated fragmentation of various dihydrochalcones and chalcones was reported. (Dihydro)cinnamic acids formed in the fragmentation showed a B-ring substitution pattern of the precursor (dihydro)chalcone. For the first time, the intrinsic generation of singlet oxygen by aspalathin and ascorbic acid under mild aqueous conditions (37 °C, pH 7.0) and exclusion of light was verified using HPLC-(+)-APCI-MS2 experiments. If a 4 molar excess of aspalathin or ascorbic acid was used, fragmentation of dihydrochalcones with monohydroxy and o-hydroxymethoxy B-ring substitution was induced up to 2 mol %, respectively. Incubations of the dihydrochalcone phloretin with ascorbic acid not only led to p-dihydrocoumaric acid but also to a novel ascorbyl adduct, which was isolated and identified as 2,4,6-trihydroxy-5-[3-(4-hydroxyphenyl)propanoyl]-2-[(1R, 2S)-1,2,3-trihydroxypropyl]-1-benzofuran-3(2H)-one. The impact of different structural elements on adduct formation was evaluated and verified to be a phloroglucinol structure linked to an acyl moiety. Formation of the ascorbyl adduct was shown to occur in apple puree when both ascorbic acid and phloretin were present at the same time.
Keywords: apple; ascorbic acid; aspalathin; chalcone; dihydrochalcone; oxidative fragmentation; phloretin; singlet oxygen.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Oxidative Fragmentation of Aspalathin Leads to the Formation of Dihydrocaffeic Acid and the Related Lysine Amide Adduct.J Agric Food Chem. 2020 Nov 18;68(46):13111-13120. doi: 10.1021/acs.jafc.9b07689. Epub 2020 Feb 5. J Agric Food Chem. 2020. PMID: 32023062
-
Antioxidant Structure⁻Activity Relationship Analysis of Five Dihydrochalcones.Molecules. 2018 May 12;23(5):1162. doi: 10.3390/molecules23051162. Molecules. 2018. PMID: 29757201 Free PMC article.
-
Oxidation of the dihydrochalcone aspalathin leads to dimerization.J Agric Food Chem. 2009 Aug 12;57(15):6838-43. doi: 10.1021/jf901614y. J Agric Food Chem. 2009. PMID: 19601579
-
Aspalathin from Rooibos (Aspalathus linearis): A Bioactive C-glucosyl Dihydrochalcone with Potential to Target the Metabolic Syndrome.Planta Med. 2018 Jul;84(9-10):568-583. doi: 10.1055/s-0044-100622. Epub 2018 Jan 31. Planta Med. 2018. PMID: 29388183 Review.
-
Biochemical Basis of Anti-Cancer-Effects of Phloretin-A Natural Dihydrochalcone.Molecules. 2019 Jan 13;24(2):278. doi: 10.3390/molecules24020278. Molecules. 2019. PMID: 30642127 Free PMC article. Review.
References
-
- Rice-Evans C.; Miller N.; Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2 (4), 152–159. 10.1016/S1360-1385(97)01018-2. - DOI
-
- Edenharder R.; von Petersdorff I.; Rauscher R. Antimutagenic effects of flavonoids, chalcones and structurally related compounds on the activity of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and other heterocyclic amine mutagens from cooked food. Mutat. Res. 1993, 287 (2), 261–274. 10.1016/0027-5107(93)90019-c. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

