Isolation of a more aggressive GVI-1 genotype strain HX of the avian infectious bronchitis virus
- PMID: 39326178
- PMCID: PMC11459636
- DOI: 10.1016/j.psj.2024.104285
Isolation of a more aggressive GVI-1 genotype strain HX of the avian infectious bronchitis virus
Abstract
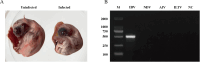
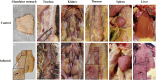
The avian infectious bronchitis virus (IBV) poses a significant economic threat to the global poultry industry. Although in recent years, the GVI-1 lineage of IBV has proliferated throughout China, there is still a lack of comprehensive studies regarding the pathogenicity of this lineage, particularly with respect to infections of the digestive tract and the antigenic characteristics of the S1 gene. In this study, we investigated the effects of infecting 14-day-old chicks with the HX strain of the GVI-1 lineage over a 14-d period postinfection. Assessment of the pathogenicity of the HX strain included clinical observations; monitoring of body weight, organ viral load, viral shedding, and gross anatomy; histopathological analysis, and bioinformatics-based antigenic characterization of the S1 protein. The findings revealed that compared with previously reported GVI-1 lineage strains, the HX strain is characterized by greater virulence, with infection leading to approximately 26% mortality and extensive severe organ damage, including that of the proventriculus and kidneys. Moreover, at 14 d postinfection, 80% of oral swabs and 100% of cloacal swabs from chickens infected with the HX strain tested positive, indicating a prolonged period of viral shedding relative to that previously reported for GVI-1 lineage strains. Bioinformatic analysis of B-cell epitopes on the S1 protein revealed 7 potential antigenic epitopes. Collectively, our findings in this study provide clear evidence to indicate that compared previously reported GVI-1 lineage strains, chicks infected with the IBV GVI-1 lineage strain HX are characterized by heightened rates of mortality, more pronounced organ damage, and an extended period of viral shedding. This comprehensive characterization highlights the pathogenic potential of the GVI-1 lineage and its capacity to induce severe kidney and proventriculus damage, thereby emphasizing the imperative of early initiated preventive measures. Furthermore, on the basis of our analysis of the antigenic properties of the S1 protein, we have identified 7 potential linear B-cell epitopes, which will provide valuable insights for the development of epitope-based vaccines.
Keywords: GVI-1; HX; S1; antigenicity; infectious bronchitis virus.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURES The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Limei Qin reports financial support was provided by Guangdong Basic and Applied Basic Research Foundation. Limei Qin reports financial support was provided by Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Efficacy of an optimal vaccination strategy for H120 and NNA vaccines against the novel HX strain of the IBV GVI-1 genotype.Vet Microbiol. 2025 Sep;308:110626. doi: 10.1016/j.vetmic.2025.110626. Epub 2025 Jul 5. Vet Microbiol. 2025. PMID: 40633273
-
Characterization of the complete genome, antigenicity, pathogenicity, tissue tropism, and shedding of a recombinant avian infectious bronchitis virus with a ck/CH/LJL/140901-like backbone and an S2 fragment from a 4/91-like virus.Virus Res. 2018 Jan 15;244:99-109. doi: 10.1016/j.virusres.2017.11.007. Epub 2017 Nov 12. Virus Res. 2018. PMID: 29141204 Free PMC article.
-
Assessing a respiratory toxic infectious bronchitis virus (IBV) strain: isolation, identification, pathogenicity, and immunological failure insights.Microbiol Spectr. 2024 Aug 6;12(8):e0399023. doi: 10.1128/spectrum.03990-23. Epub 2024 Jun 21. Microbiol Spectr. 2024. PMID: 38904372 Free PMC article.
-
Avian infectious bronchitis virus.Rev Sci Tech. 2000 Aug;19(2):493-508. doi: 10.20506/rst.19.2.1228. Rev Sci Tech. 2000. PMID: 10935276 Review.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
Lipid nanoparticles-mRNA based on the consensus sequences of avian coronavirus S1 and N genes protect animals against multiple viral infections.J Nanobiotechnology. 2025 Aug 30;23(1):595. doi: 10.1186/s12951-025-03668-5. J Nanobiotechnology. 2025. PMID: 40883775 Free PMC article.
References
-
- Abozeid H.H. Global emergence of infectious bronchitis virus variants: evolution, immunity, and vaccination challenges. Transbound. Emerg. Dis. 2023;2023:1–28.
-
- Cavanagh D., Davis P.J., Cook J.K., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. - PubMed
-
- Cavanagh M.C., Larsen R.E., Schwartz B.J. Watching Na atoms solvate into (Na+,e-) contact pairs: untangling the ultrafast charge-transfer-to-solvent dynamics of Na- in tetrahydrofuran (THF) J. Phys. Chem. 2007;111:5144–5157. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

