A PD-1-targeted, receptor-masked IL-2 immunocytokine that engages IL-2Rα strengthens T cell-mediated anti-tumor therapies
- PMID: 39326410
- PMCID: PMC11513833
- DOI: 10.1016/j.xcrm.2024.101747
A PD-1-targeted, receptor-masked IL-2 immunocytokine that engages IL-2Rα strengthens T cell-mediated anti-tumor therapies
Abstract
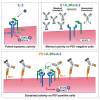
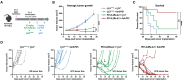
The clinical use of interleukin-2 (IL-2) for cancer immunotherapy is limited by severe toxicity. Emerging IL-2 therapies with reduced IL-2 receptor alpha (IL-2Rα) binding aim to mitigate toxicity and regulatory T cell (Treg) expansion but have had limited clinical success. Here, we show that IL-2Rα engagement is critical for the anti-tumor activity of systemic IL-2 therapy. A "non-α" IL-2 mutein induces systemic expansion of CD8+ T cells and natural killer (NK) cells over Tregs but exhibits limited anti-tumor efficacy. We develop a programmed cell death protein 1 (PD-1)-targeted, receptor-masked IL-2 immunocytokine, PD1-IL2Ra-IL2, which attenuates systemic IL-2 activity while maintaining the capacity to engage IL-2Rα on PD-1+ T cells. Mice treated with PD1-IL2Ra-IL2 show no systemic toxicities observed with unmasked IL-2 treatment yet achieve robust tumor growth control. Furthermore, PD1-IL2Ra-IL2 can be effectively combined with other T cell-mediated immunotherapies to enhance anti-tumor responses. These findings highlight the therapeutic potential of PD1-IL2Ra-IL2 as a targeted, receptor-masked, and "α-maintained" IL-2 therapy for cancer.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors are employees of Regeneron Pharmaceuticals, Inc. and may hold stock or stock options in the company. J.W., T.Z., E.S., and J.C.L. are inventors on US patent US11725034B2; “IL2 agonists and methods of uses thereof”. J.W., N.B., T.Z., J.C.L., S.D., E.S., and E.U. are inventors on a pending US patent application (US20220402989A1; “IL2-based therapeutics and methods of uses thereof”). D.J.D. and J.W. are inventors on a pending US patent application (US20240148867A1; “Methods of treating cancer with a combination of adoptive cell therapy and a targeted immunocytokine”).
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

