Improving Specificity for Ovarian Cancer Screening Using a Novel Extracellular Vesicle-Based Blood Test: Performance in a Training and Verification Cohort
- PMID: 39326669
- PMCID: PMC11600309
- DOI: 10.1016/j.jmoldx.2024.09.001
Improving Specificity for Ovarian Cancer Screening Using a Novel Extracellular Vesicle-Based Blood Test: Performance in a Training and Verification Cohort
Abstract
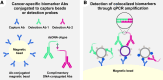
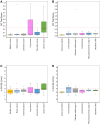
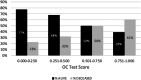
The low incidence of ovarian cancer (OC) dictates that any screening strategy needs to be both highly sensitive and highly specific. This study explored the utility of detecting multiple colocalized proteins or glycosylation epitopes on single tumor-associated extracellular vesicles from blood. The novel Mercy Halo Ovarian Cancer Test (OC Test) uses immunoaffinity capture of tumor-associated extracellular vesicles, followed by proximity-ligation real-time quantitative PCR to detect combinations of up to three biomarkers to maximize specificity, and measures multiple combinations to maximize sensitivity. A high-grade serous carcinoma (HGSC) case-control training set of EDTA plasma samples from 397 women was used to lock down the test design, the data interpretation algorithm, and the cutoff between cancer and noncancer. Performance was verified and compared with cancer antigen 125 in an independent blinded case-control set of serum samples from 390 women (132 controls, 66 HGSC, 83 non-HGSC OC, and 109 benign). In the verification study, the OC Test showed a specificity of 97.0% (128/132; 95% CI, 92.4%-99.6%), a HGSC sensitivity of 97.0% (64/66; 95% CI, 87.8%-99.2%), and an area under the curve of 0.97 (95% CI, 0.93-0.99) and detected 73.5% (61/83; 95% CI, 62.7%-82.6%) of the non-HGSC OC cases. This test exhibited fewer false positives in subjects with benign ovarian tumors, nonovarian cancers, and inflammatory conditions when compared with cancer antigen 125. The combined sensitivity and specificity of this new test suggests that it may have potential in OC screening.
Copyright © 2024 Association for Molecular Pathology and American Society for Investigative Pathology. All rights reserved.
Conflict of interest statement
Disclosure Statement L.T.B., A.D.C., D.P.S., S.B., I.O.Z., D.M.B., M.S.K., L.T.C., B.J.M., T.B.H., T.G., and D.M. are current employees of Mercy BioAnalytics Inc. E.S.W.-D. is a retired Mercy BioAnalytics employee and is currently a paid consultant of Mercy BioAnalytics Inc. She was a full-time employee when this work was performed. K.M.B., P.A.D., J.G., D.G., B.F.H., and C.R.S. are former employees of Mercy BioAnalytics Inc., who were active employees at the time this work was performed. S.J.S. and K.C. are paid consultants for Mercy BioAnalytics Inc. A.J., J.N.M., and D.H. are employees of University of British Columbia and provided the patient samples used for the training study. A.G.-M., S.A., and U.M. are employees of University College London and provided the patient samples used for the verification study. They also report research collaborations with Cambridge University, QIMR Berghofer Medical Research Institute, Intelligent Lab on Fiber, RNA Guardian, Micronoma, Imperial College London, University of Innsbruck, and Dana Farber USA in the area of early detection of cancer. U.M. had stock ownership (2011 to 2021) awarded by University College London in Abcodia, which held the license for the Risk of Ovarian Cancer Algorithm. She has received grant funding from the Medical Research Council, Cancer Research UK, the National Institute for Health Research UK, the Eve Appeal, and the Australian National Health and Medical Research Council. She is also a member of Tina's Wish Scientific Advisory Board (United States) and the Research Advisory Panel, Yorkshire Cancer Research (United Kingdom). L.T.B. and D.P.S. are inventors on US patent number 11,085,089 B2, Systems, Compositions and Methods for Target Entity Detection (issued August 10, 2021). L.T.B., D.P.S., E.S.W.-D., D.G., K.M.B., and A.D.C. are inventors on US patent application 63/417309, Composition and Methods for Detection of Ovarian Cancer (filed October 18, 2022). U.M. holds patent number EP10178345.4 for Breast Cancer Diagnostics.
Figures

References
-
- American Cancer Society: Cancer Facts & Figures 2024. American Cancer Society; Atlanta, GA: 2024.
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Alsop K., Fereday S., Meldrum C., deFazio A., Emmanuel C., George J., Dobrovic A., Birrer M.J., Webb P.M., Stewart C., Friedlander M., Fox S., Botwell D., Mitchell G. BRCA mutation frequency and patterns of treatment response in brca mutation-positive women with ovarian cancer: a report from the Australian ovarian cancer study group. J Clin Oncol. 2012;30:2654–2663. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

