Common and divergent pathways in early stages of glutamate and tau-mediated toxicities in neurodegeneration
- PMID: 39326823
- PMCID: PMC12670583
- DOI: 10.1016/j.expneurol.2024.114967
Common and divergent pathways in early stages of glutamate and tau-mediated toxicities in neurodegeneration
Abstract
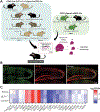
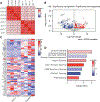
It has been shown that excitotoxicity and tau-mediated toxicities are major contributing factors to neuronal death in Alzheimer's disease (AD). The excitatory amino acid transporter 2 (EAAT2 or GLT-1), the major glutamate transporter in the brain that regulates glutamate levels synaptically and extrasynaptically, has been shown to be deficient in AD brains, leading to excitotoxicity and subsequent cell death. Similarly, buildup of neurofibrillary tangles, which consist of hyperphosphorylated tau protein, correlates with cognitive decline and neuronal atrophy in AD. However, common genes and pathways that are critical in the aforementioned toxicities have not been well elucidated. To investigate the impact of glutamate dyshomeostasis and tau accumulation on translational profiles of affected hippocampal neurons, we used mouse models of excitotoxicity and tau-mediated toxicities (GLT-1-/- and P301S, respectively) in conjunction with BAC-TRAP technology. Our data show that GLT-1 deficiency in CA3 pyramidal neurons leads to translational signatures characterized by dysregulation of pathways associated with synaptic plasticity and neuronal survival, while the P301S mutation induces changes in endocytic pathways and mitochondrial dysfunction. Finally, the commonly dysregulated pathways include impaired ion homeostasis and metabolic pathways. These common pathways may shed light on potential therapeutic targets for ameliorating glutamate and tau-mediated toxicities in AD.
Keywords: Alzheimer's disease; Bacterial artificial chromosome; Differentially expressed genes; Excitatory amino acid transporter-1; Glutamate transporter-1; Neurofibrillary tangle; Translating ribosome affinity purification.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Unrelated to this work, A.C.P. has patents unrelated to this work licensed to Neurobiopharma, LLC, serves on the scientific advisory board of Sinaptica Therapeutics and has served as a consultant to Eisai.
Figures


References
-
- Abbott Geoffrey W., 2014. Biology of the KCNQ1 Potassium Channel. New J. Sci. 2014, 237431.
-
- Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM, 2002. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am. J. Psychiatry 159, 738–745. - PubMed
-
- Barthélemy NR, Li Y, Joseph-Mathurin N, Gordon BA, Hassenstab J, Benzinger TLS, Buckles V, Fagan AM, Perrin RJ, Goate AM, Morris JC, Karch CM, Xiong C, Allegri R, Mendez PC, Berman SB, Ikeuchi T, Mori H, Shimada H, Shoji M, Suzuki K, Noble J, Farlow M, Chhatwal J, Graff-Radford NR, Salloway S, Schofield PR, Masters CL, Martins RN, O’Connor A, Fox NC, Levin J, Jucker M, Gabelle A, Lehmann S, Sato C, Bateman RJ, McDade E, 2020. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 26, 398–407. - PMC - PubMed
-
- Bi X, Gall CM, Zhou J, Lynch G, 2002. Uptake and pathogenic effects of amyloid beta peptide 1–42 are enhanced by integrin antagonists and blocked by NMDA receptor antagonists. Neuroscience 112, 827–840. - PubMed
-
- Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW, 2005. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta 1739, 216–223. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

