Efficacy and safety of selexipag in patients with inoperable or persistent/recurrent CTEPH (SELECT randomised trial)
- PMID: 39326918
- PMCID: PMC11447286
- DOI: 10.1183/13993003.00193-2024
Efficacy and safety of selexipag in patients with inoperable or persistent/recurrent CTEPH (SELECT randomised trial)
Abstract
Background: SELECT was the first global randomised controlled trial of selexipag with standard of care in patients with inoperable or persistent/recurrent chronic thromboembolic pulmonary hypertension.
Methods: SELECT was a multicentre, randomised, double-blind, placebo-controlled, parallel-group, group-sequential, phase 3 study (ClinicalTrials.gov: NCT03689244). Adults aged ≤85 years in World Health Organization Functional Class I-IV, with a 6-min walk distance of 100-450 m, were randomised (1:1) to receive selexipag (200-1600 µg twice daily titration until individual maximum tolerated dose)+standard of care or placebo+standard of care. Patients were recruited into the haemodynamic set (first 91 randomised patients to undergo right heart catheterisation (RHC); week 20) or non-haemodynamic cohort (remaining patients, no RHC required). The primary end-point was percent of baseline pulmonary vascular resistance (PVR; week 20). Safety was also assessed.
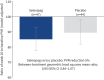
Results: Of 321 patients screened, 128 were randomised (haemodynamic set n=91 (selexipag n=47; placebo n=44)). In the haemodynamic set, 29 (31.9%) patients had previous pulmonary endarterectomy (PEA), 20 (22.0%) balloon pulmonary angioplasty (BPA), and 14 (15.4%) both PEA and BPA; 28 (30.8%) were inoperable. The independent data monitoring committee recommended to stop the study for futility as no statistically significant difference was observed for the primary end-point (between-treatment geometric least squares mean ratio of PVR: 0.95, 95% CI 0.84-1.07; p=0.412). Adverse events were reported in 63 (98.4%) and 53 (82.8%) patients for selexipag and placebo, respectively.
Conclusions: SELECT was discontinued for futility, as no treatment effect on the primary end-point (PVR) was observed. Safety data were consistent with the established safety profile of selexipag, with no new safety signals identified.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: N.H. Kim has consulted for, received research grants from or spoken for Janssen, Bayer, Merck, United Therapeutics, Enzyvant, Gossamer Bio, Polarean and Pulnovo. R. Channick has consulted for, received research grants from or spoken for Janssen, Bayer, United Therapeutics, Respira, Third Pole, Merck, Aria CV and Gossamer. M. Delcroix reports research grants from Janssen, speaker and consultant fees from Altavant, Acceleron, AOP, Bayer, Ferrer, Gossamer, INARI, Janssen, United Therapeutics and MSD, outside the submitted work, and all paid to her institution; and is holder of a Janssen Chair for Pulmonary Hypertension at KU Leuven. M. Madani has served as a consultant and has received fees from Wexler Surgical, Janssen, Bayer and MSD. J. Pepke-Zaba reports research grants from MSD and consultant fees from Ferrer, Gossamer, Janssen, United Therapeutics and MSD. V. Easton, S. Gesang and D. Richard are employees of Johnson & Johnson and own shares in the company. J.I. Borissoff is an employee of Johnson & Johnson. H-A. Ghofrani reports consultancy fees from Gossamer Bio, Inc., Aerovate, Altavant, Bayer AG, Attgeno, Janssen/Actelion, MSD/Acceleron, Pfizer, Liquidia, Morphic and Keros, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer AG, Janssen/Actelion, Gossamer Bio, Keros and MSD/Acceleron, participation on a data safety monitoring board or advisory board with Aerovate, Altavant, Bayer AG, Attgeno, Janssen/Actelion, Insmed, MSD/Acceleron and Pfizer; H-A. Ghofrani's spouse is an employee of Liquidia.
Figures


Comment in
-
Selexipag: still looking for its place.Eur Respir J. 2024 Oct 3;64(4):2401560. doi: 10.1183/13993003.01560-2024. Print 2024 Oct. Eur Respir J. 2024. PMID: 39362684 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
