Monosynaptic Inputs to Ventral Tegmental Area Glutamate and GABA Co-transmitting Neurons
- PMID: 39327007
- PMCID: PMC11561872
- DOI: 10.1523/JNEUROSCI.2184-23.2024
Monosynaptic Inputs to Ventral Tegmental Area Glutamate and GABA Co-transmitting Neurons
Abstract
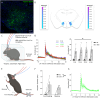
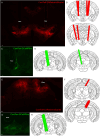
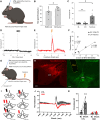
A unique population of ventral tegmental area (VTA) neurons co-transmits glutamate and GABA. However, the circuit inputs to VTA VGluT2+VGaT+ neurons are unknown, limiting our understanding of their functional capabilities. By coupling monosynaptic rabies tracing with intersectional genetic targeting in male and female mice, we found that VTA VGluT2+VGaT+ neurons received diverse brainwide inputs. The largest numbers of monosynaptic inputs to VTA VGluT2+VGaT+ neurons were from superior colliculus (SC), lateral hypothalamus (LH), midbrain reticular nucleus, and periaqueductal gray, whereas the densest inputs relative to brain region volume were from the dorsal raphe nucleus, lateral habenula, and VTA. Based on these and prior data, we hypothesized that LH and SC inputs were from glutamatergic neurons. Optical activation of glutamatergic LH neurons activated VTA VGluT2+VGaT+ neurons regardless of stimulation frequency and resulted in flee-like ambulatory behavior. In contrast, optical activation of glutamatergic SC neurons activated VTA VGluT2+VGaT+ neurons for a brief period of time at high frequency and resulted in head rotation and arrested ambulatory behavior (freezing). Stimulation of glutamatergic LH neurons, but not glutamatergic SC neurons, was associated with VTA VGluT2+VGaT+ footshock-induced activity and inhibition of LH glutamatergic neurons disrupted VTA VGluT2+VGaT+ tailshock-induced activity. We interpret these results such that inputs to VTA VGluT2+VGaT+ neurons may integrate diverse signals related to the detection and processing of motivationally salient outcomes.
Keywords: VTA VGluT2 neurons; co-transmit; colliculus; hypothalamus; threat; vesicular glutamate transporter 2.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Update of
-
Monosynaptic inputs to ventral tegmental area glutamate and GABA co-transmitting neurons.bioRxiv [Preprint]. 2023 Nov 21:2023.04.06.535959. doi: 10.1101/2023.04.06.535959. bioRxiv. 2023. Update in: J Neurosci. 2024 Nov 13;44(46):e2184232024. doi: 10.1523/JNEUROSCI.2184-23.2024. PMID: 37066408 Free PMC article. Updated. Preprint.
References
-
- Barbano MF, Zhang S, Chen E, Espinoza O, Mohammad U, Alvarez-Bagnarol Y, Liu B, Hahn S, Morales M (2024) Lateral hypothalamic glutamatergic inputs to VTA glutamatergic neurons mediate prioritization of innate defensive behavior over feeding. Nat Commun 15:403. 10.1038/s41467-023-44633-w - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
