EphB2 Signaling Is Implicated in Astrocyte-Mediated Parvalbumin Inhibitory Synapse Development
- PMID: 39327008
- PMCID: PMC11551896
- DOI: 10.1523/JNEUROSCI.0154-24.2024
EphB2 Signaling Is Implicated in Astrocyte-Mediated Parvalbumin Inhibitory Synapse Development
Abstract
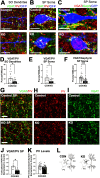
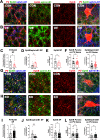
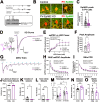
Impaired inhibitory synapse development is suggested to drive neuronal hyperactivity in autism spectrum disorders (ASD) and epilepsy. We propose a novel mechanism by which astrocytes control the development of parvalbumin (PV)-specific inhibitory synapses in the hippocampus, implicating ephrin-B/EphB signaling. Here, we utilize genetic approaches to assess functional and structural connectivity between PV and pyramidal cells (PCs) through whole-cell patch-clamp electrophysiology, optogenetics, immunohistochemical analysis, and behaviors in male and female mice. While inhibitory synapse development is adversely affected by PV-specific expression of EphB2, a strong candidate ASD risk gene, astrocytic ephrin-B1 facilitates PV→PC connectivity through a mechanism involving EphB signaling in PV boutons. In contrast, the loss of astrocytic ephrin-B1 reduces PV→PC connectivity and inhibition, resulting in increased seizure susceptibility and an ASD-like phenotype. Our findings underscore the crucial role of astrocytes in regulating inhibitory circuit development and discover a new role of EphB2 receptors in PV-specific inhibitory synapse development.
Keywords: EphB receptor; astrocyte; hippocampus; inhibition; parvalbumin; synapse.
Copyright © 2024 Sutley-Koury et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Association AP (2013) Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Publishing.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
