Widespread occurrence of dissolved oxygen anomalies, aerobic microbes, and oxygen-producing metabolic pathways in apparently anoxic environments
- PMID: 39327011
- PMCID: PMC11549561
- DOI: 10.1093/femsec/fiae132
Widespread occurrence of dissolved oxygen anomalies, aerobic microbes, and oxygen-producing metabolic pathways in apparently anoxic environments
Abstract
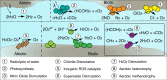
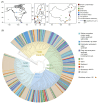
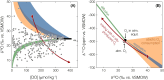
Nearly all molecular oxygen (O2) on Earth is produced via oxygenic photosynthesis by plants or photosynthetically active microorganisms. Light-independent O2 production, which occurs both abiotically, e.g. through water radiolysis, or biotically, e.g. through the dismutation of nitric oxide or chlorite, has been thought to be negligible to the Earth system. However, recent work indicates that O2 is produced and consumed in dark and apparently anoxic environments at a much larger scale than assumed. Studies have shown that isotopically light O2 can accumulate in old groundwaters, that strictly aerobic microorganisms are present in many apparently anoxic habitats, and that microbes and metabolisms that can produce O2 without light are widespread and abundant in diverse ecosystems. Analysis of published metagenomic data reveals that the enzyme putatively capable of nitric oxide dismutation forms four major phylogenetic clusters and occurs in at least 16 bacterial phyla, most notably the Bacteroidota. Similarly, a re-analysis of published isotopic signatures of dissolved O2 in groundwater suggests in situ production in up to half of the studied environments. Geochemical and microbiological data support the conclusion that "dark oxygen production" is an important and widespread yet overlooked process in apparently anoxic environments with far-reaching implications for subsurface biogeochemistry and ecology.
Keywords: chlorite dismutation; cryptic O2 cycling; dark oxygen production; hypoxia; nitric oxide dismutation; subsurface microbiome.
© The Author(s) 2024. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures



References
-
- Aburto A, Fahy A, Coulon F et al. Mixed aerobic and anaerobic microbial communities in benzene-contaminated groundwater. J Appl Microbiol. 2009;106:317–28. - PubMed
-
- Achenbach LA, Michaelidou U, Bruce RA et al. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int J Syst Evol Microbiol. 2001;51:527–33. - PubMed
-
- Aggarwal PK, Dillon MA. Stable isotope composition of molecular oxygen in soil gas and groundwater: a potentially robust tracer for diffusion and oxygen consumption processes. Geochim Cosmochim Acta. 1998;62:577–84.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

