An Integrated Approach for Representing Knowledge on the Potential of Drugs to Cause Acute Kidney Injury
- PMID: 39327387
- PMCID: PMC11711143
- DOI: 10.1007/s40264-024-01474-w
An Integrated Approach for Representing Knowledge on the Potential of Drugs to Cause Acute Kidney Injury
Abstract
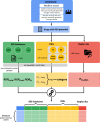
Introduction and objective: The recent rise in acute kidney injury (AKI) incidence, with approximately 30% attributed to potentially preventable adverse drug events (ADEs), poses challenges in evaluating drug-induced AKI due to polypharmacy and other risk factors. This study seeks to consolidate knowledge on the drugs with AKI potential from four distinct sources: (i) bio(medical) peer-reviewed journals; (ii) spontaneous reporting systems (SRS); (iii) drug information databases (DIDs); and (iv) NephroTox website. By harnessing the potential of these underutilised sources, our objective is to bridge gaps and enhance the understanding of drug-induced AKI.
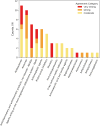
Methods: By searching Medline, studies with lists of drugs with AKI potential established through consensus amongst medical experts were selected. A final list of 63 drugs was generated aggregating the original studies. For these 63 drugs, the AKI reporting odds ratios (RORs) using three SRS databases, the average frequency of ADEs from four different DIDs and the number of published studies identified via NephroTox was reported.
Results: Drugs belonging to the antivirals, antibacterials, and non-steroidal anti-inflammatory pharmacological classes exhibit substantial consensus on AKI potential, which was also reflected in strong ROR signals, frequent to very frequent AKI-related ADEs and a high number of published studies reporting adverse kidney events as identified via NephroTox. Renin-angiotensin aldosterone system inhibitors and diuretics also display comparable signal strengths, but this can be attributed to expected haemodynamic changes. More variability is noted for proton-pump inhibitors.
Conclusions: By integrating four disjointed sources of knowledge, we have created a novel, comprehensive resource on drugs with AKI potential, contributing to kidney safety improvement efforts.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: This study is part of a larger project called LEveraging real-world dAta to optimize PharmacotheRapy outcomes in multimOrbid patients by using machine learning and knowledGe representation methods (LEAPfROG project) funded by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO; Dutch Research Council) under grant agreement KICH1.ST01.20.011 and co-funded in-cash by Dutch Kidney Foundation and National Intensive Care Evaluation (NICE) foundation, and in-kind by PHARMO Institute for Drug Outcomes Research, Castor, InsightRX, Z-Index, Digital Health Link. The funders had no role in the design of the study, the collection, analysis and interpretation of the data, or in writing the manuscript. Conflict of interest: Daniel Fernández-Llaneza is a former employee of AstraZeneca and terminated his contract in February 2023. In the last 3 years, Dr. T.vG. has received lecture fees and consulting fees from Roche Diagnostics, Thermo Fisher, Vitaeris, Otsuka, CSL Behring, Astellas and Aurinia Pharma. In all cases money has been transferred to hospital accounts, and none has been paid to his personal bank accounts. Dr. T.vG. does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. Dr. S.L.K-G. receives grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases R01DK121730 and U01DK130010, the National Center for Complementary and Integrative Health U54AT008909 and the Jewish Healthcare Foundation. All other authors declared no competing interests for this work. Availability of data and material: Some of the data supporting the findings of this study are not publicly available. Access to EV data is restricted based on conditions for access specified in EMA’s access policy ( https://www.adrreports.eu/en/access_policy.html ). Micromedex® and Informatorium Medicamentorum data was accessed through the hospital’s licence. All publications, DAEN and CVAR SRS databases data and SIDER and FK data are freely and publicly accessible. Ethics approval: This study is part of a larger project called LEveraging real-world dAta to optimize PharmacotheRapy outcomes in multimOrbid patients by using machine learning and knowledGe representation methods (LEAPfROG project).The LEAPfROG project was exempted from requiring ethics approval (waiver W22_340 # 22.412) on September 2022, 22 by the Medical Ethics Committee of the Amsterdam University Medical Centre, University of Amsterdam, Amsterdam, The Netherlands, as it did not fall within the scope of the Dutch Medical Research Involving Human Subjects Act. The anonymised ICSR data elements of DAEN and CVAR are available as an online open source. In order to get an extended subset of ICSR data elements from EV (level 2A), a research request and a signed copy of a confidentiality undertaking were submitted to the EMA. These were then presented to the EV research committee, which granted access to the data. This does not affect the rights to publish the results. Consent to participate: Not applicable. Consent for publication: Not applicable. Code availability: Code is available upon reasonable request to the authors. Author contributions (CRediT taxonomy): Daniel Fernández-Llaneza: Conceptualisation, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation; Writing—original draft, Visualisation; Romy M. P Vos: Conceptualisation, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation; Writing—original draft, Visualisation; Joris E. Lieverse: Conceptualisation, Methodology, Validation, Formal analysis, Investigation, Data Curation; Writing – original draft, Visualisation; Helen R. Gosselt, Sandra L. Kane-Gill, Teun van Gelder: Methodology; Validation; Writing—review and editing; Joanna E. Klopotowska: Conceptualisation; Data Curation; Formal Analysis; Funding Acquisition; Methodology; Project Administration; Validation; Writing—review and editing. All authors and LEAPfROG consortium members gave final approval of the submitted version.
Figures
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1).
-
- Cerdá J, et al. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4(3):138–53. 10.1038/ncpneph0722. - PubMed
-
- Hoste EAJ, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23. 10.1007/s00134-015-3934-7. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

