Characterization of ferroptosis-triggered pyroptotic signaling in heart failure
- PMID: 39327446
- PMCID: PMC11427671
- DOI: 10.1038/s41392-024-01962-6
Characterization of ferroptosis-triggered pyroptotic signaling in heart failure
Abstract
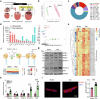
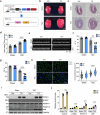
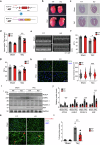
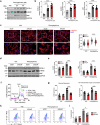
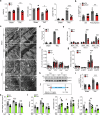
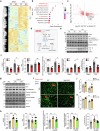
Pressure overload-induced cardiac hypertrophy is a common cause of heart failure (HF), and emerging evidence suggests that excessive oxidized lipids have a detrimental effect on cardiomyocytes. However, the key regulator of lipid toxicity in cardiomyocytes during this pathological process remains unknown. Here, we used lipidomics profiling and RNA-seq analysis and found that phosphatidylethanolamines (PEs) and Acsl4 expression are significantly increased in mice with transverse aortic constriction (TAC)-induced HF compared to sham-operated mice. In addition, we found that overexpressing Acsl4 in cardiomyocytes exacerbates pressure overload‒induced cardiac dysfunction via ferroptosis. Notably, both pharmacological inhibition and genetic deletion of Acsl4 significantly reduced left ventricular chamber size and improved cardiac function in mice with TAC-induced HF. Moreover, silencing Acsl4 expression in cultured neonatal rat ventricular myocytes was sufficient to inhibit hypertrophic stimulus‒induced cell growth. Mechanistically, we found that Acsl4-dependent ferroptosis activates the pyroptotic signaling pathway, which leads to increased production of the proinflammatory cytokine IL-1β, and neutralizing IL-1β improved cardiac function in Acsl4 transgenic mice following TAC. These results indicate that ACSL4 plays an essential role in the heart during pressure overload‒induced cardiac remodeling via ferroptosis-induced pyroptotic signaling. Together, these findings provide compelling evidence that targeting the ACSL4-ferroptosis-pyroptotic signaling cascade may provide a promising therapeutic strategy for preventing heart failure.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation145, e895–e1032 (2022). - PubMed
-
- Tsao, C. W. et al. Heart Disease and Stroke Statistics-2022 update: a report from the American Heart Association. Circulation145, e153–e639 (2022). - PubMed
-
- McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.42, 3599–3726 (2021). - PubMed
-
- Bertero, E. & Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol.15, 457–470 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 32330047/National Natural Science Foundation of China (National Science Foundation of China)
- 81900232/National Natural Science Foundation of China (National Science Foundation of China)
- 81800706/National Natural Science Foundation of China (National Science Foundation of China)
- 31970689/National Natural Science Foundation of China (National Science Foundation of China)
- LQ19H020011/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

