Immunomodulation in dengue: towards deciphering dengue severity markers
- PMID: 39327552
- PMCID: PMC11425918
- DOI: 10.1186/s12964-024-01779-4
Immunomodulation in dengue: towards deciphering dengue severity markers
Abstract
Background: Dengue is a vector-borne debilitating disease that is manifested as mild dengue fever, dengue with warning signs, and severe dengue. Dengue infection provokes a collective immune response; in particular, the innate immune response plays a key role in primary infection and adaptive immunity during secondary infection. In this review, we comprehensively walk through the various markers of immune response against dengue pathogenesis and outcome.
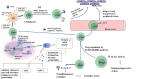
Main body: Innate immune response against dengue involves a collective response through the expression of proinflammatory cytokines, such as tumor necrosis factors (TNFs), interferons (IFNs), and interleukins (ILs), in addition to anti-inflammatory cytokines and toll-like receptors (TLRs) in modulating viral pathogenesis. Monocytes, dendritic cells (DCs), and mast cells are the primary innate immune cells initially infected by DENV. Such immune cells modulate the expression of various markers, which can influence disease severity by aiding virus entry and proinflammatory responses. Adaptive immune response is mainly aided by B and T lymphocytes, which stimulate the formation of germinal centers for plasmablast development and antibody production. Such antibodies are serotype-dependent and can aid in virus entry during secondary infection, mediated through a different serotype, such as in antibody-dependent enhancement (ADE), leading to DENV severity. The entire immunological repertoire is exhibited differently depending on the immune status of the individual.
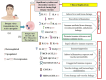
Short conclusion: Dengue fever through severe dengue proceeds along with the modulated expression of several immune markers. In particular, TLR2, TNF-α, IFN-I, IL-6, IL-8, IL-17 and IL-10, in addition to intermediate monocytes (CD14+CD16+) and Th17 (CD4+IL-17+) cells are highly expressed during severe dengue. Such markers could assist greatly in severity assessment, prompt diagnosis, and treatment.
Keywords: Aedes mosquitoes; Adaptive immunity; Dengue; Immunomodulator; Innate immunity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Fong S-L, Wong K-T, Tan C-T. Dengue virus infection and neurological manifestations: an update. Brain. 2024;147:830–38. - PubMed
-
- Dengue and severe dengue [cited 2024 Apr 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

