Sex-dependent effects of acute stress and alcohol exposure during adolescence on mRNA expression of brain signaling systems involved in reward and stress responses in young adult rats
- PMID: 39327618
- PMCID: PMC11426001
- DOI: 10.1186/s13293-024-00649-5
Sex-dependent effects of acute stress and alcohol exposure during adolescence on mRNA expression of brain signaling systems involved in reward and stress responses in young adult rats
Abstract
Background: Adolescent stress and alcohol exposure increase the risk of maladaptive behaviors and mental disorders in adulthood, with distinct sex-specific differences. Understanding the mechanisms underlying these early events is crucial for developing targeted prevention and treatment strategies.
Methods: Male and female Wistar rats were exposed to acute restraint stress and intermittent alcohol during adolescence. We assessed lasting effects on plasma corticosterone (CORT) and adrenocorticotropic hormone (ACTH) levels, and mRNA expression of genes related to corticotropin releasing hormone (CRH), neuropeptide Y (NPY), corticoid, opioid, and arginine vasopressin systems in the amygdala and hypothalamus.
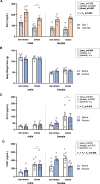
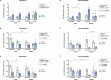
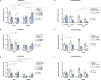
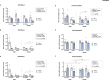
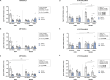
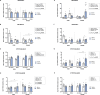
Results: The main findings are as follows: (1) blood alcohol concentrations (BAC) increased after the final alcohol administration, but stressed males had lower BAC than non-stressed males; (2) Males gained significantly more weight than females; (3) Stressed females showed higher ACTH levels than non-stressed females, with no changes in males; (4) Stress increased CORT levels in males, while stressed, alcohol-treated females had lower CORT levels than non-stressed females; (5) CRH: Females had lower Crhr1 levels in the amygdala, while alcohol reduced Crhr2 levels in males but not females. Significant interactions among sex, stress, and alcohol were found in the hypothalamus, with distinct patterns between sexes; (6) NPY: In the amygdala, stress reduced Npy and Npy1r levels in males but increased them in females. Alcohol decreased Npy2r levels in males, with varied effects in females. Similar sex-specific patterns were observed in the hypothalamus; (7) Corticoid system: Stress and alcohol had complex, sex-dependent effects on Pomc, Nr3c1, and Nr3c2 in both brain regions; (8) Opioid receptors: Stress and alcohol blunted the elevated expression of Oprm1, Oprd1, and Oprk1 in the amygdala of males and the hypothalamus of females; (8) Vasopressin: Stress and alcohol interacted significantly to affect Avp and Avpr1a expression in the amygdala, with stronger effects in females. In the hypothalamus, alcohol increased Avp levels in females.
Conclusions: This study demonstrates that adolescent acute stress and alcohol exposure induce lasting, sex-specific alterations in systems involved in reward and stress responses. These findings emphasize the importance of considering sex differences in the prevention and management of HPA dysfunction and psychiatric disorders.
Keywords: Adolescence; Amygdala; Binge drinking; HPA axis; Hypothalamus; Restraint; Sexual dimorphism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats.Psychoneuroendocrinology. 2009 Feb;34(2):226-237. doi: 10.1016/j.psyneuen.2008.09.003. Epub 2008 Oct 11. Psychoneuroendocrinology. 2009. PMID: 18849120
-
Exposure to Chronic Mild Stress Differentially Alters Corticotropin-Releasing Hormone and Arginine Vasopressin mRNA Expression in the Stress-Responsive Neurocircuitry of Male and Female Rats Prenatally Exposed to Alcohol.Alcohol Clin Exp Res. 2015 Dec;39(12):2414-21. doi: 10.1111/acer.12916. Epub 2015 Nov 18. Alcohol Clin Exp Res. 2015. PMID: 26578428 Free PMC article.
-
Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone.Alcohol Clin Exp Res. 2007 Sep;31(9):1598-610. doi: 10.1111/j.1530-0277.2007.00460.x. Alcohol Clin Exp Res. 2007. PMID: 17760789
-
Molecular and cellular sex differences at the intersection of stress and arousal.Neuropharmacology. 2012 Jan;62(1):13-20. doi: 10.1016/j.neuropharm.2011.06.004. Epub 2011 Jun 21. Neuropharmacology. 2012. PMID: 21712048 Free PMC article. Review.
-
Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin.Behav Pharmacol. 2014 Sep;25(5-6):445-57. doi: 10.1097/FBP.0000000000000049. Behav Pharmacol. 2014. PMID: 24949572 Free PMC article. Review.
Cited by
-
Anti-Hangover and Hepatoprotective Effects of the Leaf Extract of Thunbergia laurifolia in Sprague-Dawley Rats.Pharmaceuticals (Basel). 2025 May 5;18(5):685. doi: 10.3390/ph18050685. Pharmaceuticals (Basel). 2025. PMID: 40430504 Free PMC article.
References
-
- Worst TJ, Vrana KE. Alcohol and gene expression in the central nervous system. Alcohol Alcohol. 2005;40(1):63–75. - PubMed
MeSH terms
Substances
Grants and funding
- PI19/00886, PI20/01399, PI22/00427 and PI22/01833/Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación and European Regional Development Funds-European Union (ERDF-EU)
- PI19/00886, PI20/01399, PI22/00427 and PI22/01833/Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación and European Regional Development Funds-European Union (ERDF-EU)
- PI19/00886, PI20/01399, PI22/00427 and PI22/01833/Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación and European Regional Development Funds-European Union (ERDF-EU)
- PT20-00101/Plataforma de biobanco y biomodelos animales y 3D de Málaga
- PT20-00101/Plataforma de biobanco y biomodelos animales y 3D de Málaga
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

