Novel single-cell measurements suggest irreversibly sickled cells are neither dense nor dehydrated
- PMID: 39327732
- PMCID: PMC11560302
- DOI: 10.1016/j.bpj.2024.09.024
Novel single-cell measurements suggest irreversibly sickled cells are neither dense nor dehydrated
Abstract
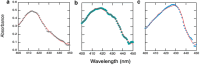
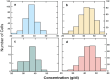
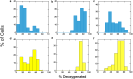
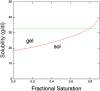
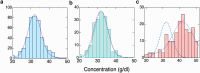
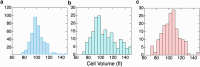
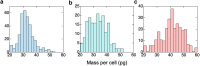
In sickle cell anemia, deoxygenation causes erythrocytes to distort, while reoxygenation permits them to recover a normal biconcave disk shape. Irreversibly sickled cells (ISCs) remain distorted when reoxygenated and have been thought to have among the highest intracellular hemoglobin concentrations of the sickle red cell population and therefore the greatest vulnerability to vasoocclusion. Using a new optical method, which we describe, we have made precise measurements of the intracellular hemoglobin concentration, and intracellular O2 saturation, of ISCs, as well as oxygenated sickle cells with a normal biconcave disc shape, and cells with shapes distorted by the sickle fibers they contain. This method also provides good estimates of cell volumes, and hemoglobin per red cell. The concentration distribution of the ISCs is found to be similar to normal, discoid cells. Average ISC volumes exceed their discoid counterparts, with a much broader distribution, arguing against dehydration as their origin. The concentration distribution of the polymer-laden sickled cells is significantly higher in mean value, and their volume distributions indicate some dehydration. Previous assumptions about ISCs may have thus been colored by the presence of sickle cells that did contain polymer, and true ISCs may be much more benign than once thought, which underscores the importance of accurate measurement on individual cells. This method could be used to follow changes in individual cell properties under various specific perturbations, and where characterization by flow cytometry is infeasible.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare no conflicts of interest.
Figures

Comment in
-
Reversing the understanding of irreversibly sickled cells.Biophys J. 2024 Nov 5;123(21):3653-3654. doi: 10.1016/j.bpj.2024.08.013. Epub 2024 Aug 22. Biophys J. 2024. PMID: 39175196 Free PMC article. No abstract available.
References
-
- Hahn E.V., Gillespie E.B. SICKLE CELL ANEMIA: REPORT OF A CASE GREATLY IMPROVED BY SPLENECTOMY. EXPERIMENTAL STUDY OF SICKLE CELL FORMATION. Arch. Intern. Med. 1927;39:233–254.
-
- Eaton W.A., Hofrichter J., Ross P.D. Delay time of gelation: a possible determinant of clinical severity in sickle cell disease. Blood. 1976;47:621–627. - PubMed
-
- Glader B.E., Nathan D.G. Cation permeability alterations during sickling: relationship to cation composition and cellular hydration of irreversibly sickled cells. Blood. 1978;51:983–989. https://www.ncbi.nlm.nih.gov/pubmed/638256 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

