Performance and Safety of the Extravascular Implantable Cardioverter Defibrillator Through Long-Term Follow-Up: Final Results From the Pivotal Study
- PMID: 39327797
- PMCID: PMC11771354
- DOI: 10.1161/CIRCULATIONAHA.124.071795
Performance and Safety of the Extravascular Implantable Cardioverter Defibrillator Through Long-Term Follow-Up: Final Results From the Pivotal Study
Abstract
Background: Substernal lead placement of the extravascular implantable cardioverter defibrillator (EV ICD) permits both defibrillation at thresholds similar to those seen with transvenous implantable cardioverter defibrillators and effective anti-tachycardia pacing (ATP) while avoiding the vasculature and associated complications. The global Pivotal study has shown the EV ICD system to be safe and effective through 6 months, but long-term experience has yet to be published. Our aim was to report the performance and safety of the EV ICD system throughout the study.
Methods: The EV ICD Pivotal study was a prospective, global, single-arm, premarket clinical study. Individuals with a Class I or IIa indication for a single-chamber implantable cardioverter defibrillator per guidelines were enrolled. Freedom from major system- or procedure-related complications and appropriate and inappropriate therapy rates were assessed through 3 years with the Kaplan-Meier method. ATP success was calculated from simple proportions.
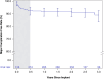
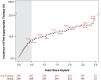
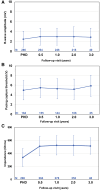
Results: An implantation was attempted in 316 patients (25.3% female; 53.8±13.1 years of age; 81.6% primary prevention; left ventricular ejection fraction, 38.9±15.4%). Of 299 patients with a successful implantation, 24 experienced 82 spontaneous arrhythmic episodes that were appropriately treated with ATP only (38, 46.3%), shock only (34, 41.5%), or both (10, 12.2%) for a Kaplan-Meier-estimated rate of first any appropriate therapy of 9.2% at 3 years. ATP was successful in 77.1% (37/48) of episodes, and ATP use significantly increased from discharge to last follow-up visit (P<0.0001). Shock therapy was successful in 100% (27/27) of discrete, spontaneous ventricular arrhythmias. The inappropriate shock rates at 1 and 3 years were 9.8% and 17.5%, respectively, with P-wave oversensing the predominant cause. No major intraprocedural complications were reported, and the estimated freedom from system- or procedure-related major complications was 91.9% at 1 year and 89.0% at 3 years. The most common major complications were lead dislodgement (10 events; n=9 patients, 2.8%), postoperative wound or device pocket infection (n=8, 2.5%), and device inappropriate shock delivery (n=4, 1.3%). Twenty-four system revisions were performed as a result of major complications related to the EV ICD system or procedure.
Conclusions: From implantation to study completion, the EV ICD Pivotal study demonstrated that a single integrated system with an extravascular lead placed in the substernal space maintains high ATP success, effective defibrillation, and a consistent safety profile.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04060680.
Keywords: arrhythmias, cardiac; cardiac pacing, artificial; death, sudden, cardiac; defibrillators, implantable; primary prevention; secondary prevention.
Conflict of interest statement
Dr Friedman reports receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic; licenses and patents with Anumana, Eko Health, Alive Cor, and Marani Health; advisory board participation at Anumana; a leadership position with xAI.health and MediCool; steering committee participation and advisory group participation at Boston Scientific, funds paid to their institution; and stock from MediCool, Marani Health, and xAI.health. Dr Murgatroyd reports receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic; consulting fees from Medtronic for advisory board and steering committee participation, consulting fees from Boston Scientific for steering committee participation; and speaker fees from Medtronic. Dr Boersma reports receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic and consulting fees from Medtronic and Adagio, paid to the cardiology department at their institution. Dr Manlucu reports receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic; consulting fees from Medtronic for advisory board and steering committee participation; and speaker fees from Medtronic. Dr Knight reports receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic; consulting fees from Medtronic for advisory board and steering committee participation; consulting fees from Boston Scientific for advisory board participation; and speaker fees from Medtronic and Boston Scientific. Dr Clémenty reports receiving consulting fees, honoraria fees, travel support, and advisory board participation from Medtronic. Dr Leclercq reports receiving consulting fees and honoraria from Medtronic, Boston Scientific, and Abbott for steering committee participation and speaker fees from Medtronic. Dr Amin reports receiving EV ICD Pivotal study participation support from Medtronic; an institutional grant from Biosense Webster; consulting fees from Medtronic, Boston Scientific, Biosense Webster, Atricure, and Philips; honoraria from Medtronic, Boston Scientific, Biosense Webster, Atricure, and Philips; travel support from Boston Scientific, Medtronic, and Philips; and advisory board participation at Boston Scientific, Medtronic, Atricure, and Philips. Dr Merkely reports receiving institutional grants from Medtronic and Boston Scientific and lecture fees from Medtronic, Biotronik, and Abbott. Dr Birgersdotter-Green reports receiving honoraria from Medtronic, Boston Scientific, Abbott, and Biotronik; advisory board participation at Biotronik; and stock ownership in Vektor Medical. Dr Chan reports receiving honoraria for lectures from Medtronic. Dr Biffi receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic and grants from Medtronic, paid to their institution. Dr Knops reports receiving EV ICD Pivotal study participation support from Medtronic for trial funding; grants from Boston Scientific and Abbott for trial funding; institutional and private consulting fees from Abbott and Boston Scientific; honoraria from Medtronic, Boston Scientific, and Abbott; advisory board participation at Boston Scientific, Atacor, and Kestra; participation on the ESC pacing guideline committee; and stock ownership from Atacor. Dr Engel reports consulting fees from Medtronic. Dr Epstein reports institutional support for the EV ICD Pivotal study, consulting fees from Medtronic, and honoraria from Medtronic. Dr Johansen reports honoraria paid to their institution from Merit Medical, Education course and advisory board participation at Medtronic and Biotronik. Dr Sterliński reports receiving EV ICD Pivotal study participation support, paid to their institution, from Medtronic; grants from Biotronik and HammerMed; consulting fees from Abbott, Biotronik, HammerMed, and Medtronic; honoraria from Abbott, Biotronik, Medtronic, and Zoll; and advisory board participation at Medtronic. Dr Hounshell reports consulting fees from Medtronic, lecture fees from Medtronic, and advisory board participation at Medtronic. Dr Abben reports consulting fees and honoraria from Medtronic. A. Thompson, Y. Zhang, Dr Wiggenhorn, and S. Willey report employment by Medtronic. Dr Crozier reports receiving research funding for the EV ICD Pivotal study from Medtronic, grants from Medtronic for fellowship funding, and consulting fees from Medtronic. The other authors report no conflicts.
Figures

References
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, et al. . Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia: Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601 - PubMed
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399 - PubMed
-
- Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926–932. doi: 10.1111/j.1540-8159.2005.00195.x - PubMed
-
- Donnelly J, Gabriels J, Galmer A, Willner J, Beldner S, Epstein LM, Patel A. Venous obstruction in cardiac rhythm device therapy. Curr Treat Options Cardiovasc Med. 2018;20:64. doi: 10.1007/s11936-018-0664-5 - PubMed
-
- Nso N, Nassar M, Lakhdar S, Enoru S, Guzman L, Rizzo V, Munira MS, Radparvar F, Thambidorai S. Comparative assessment of transvenous versus subcutaneous implantable cardioverter-defibrillator therapy outcomes: an updated systematic review and meta-analysis. Int J Cardiol. 2022;349:62–78. doi: 10.1016/j.ijcard.2021.11.029 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

