Structural basis of Pseudomonas aeruginosa penicillin binding protein 3 inhibition by the siderophore-antibiotic cefiderocol
- PMID: 39328188
- PMCID: PMC11423509
- DOI: 10.1039/d4sc04937c
Structural basis of Pseudomonas aeruginosa penicillin binding protein 3 inhibition by the siderophore-antibiotic cefiderocol
Abstract
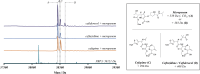
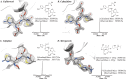
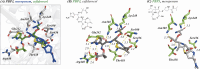
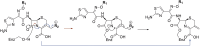
The breakthrough cephalosporin cefiderocol, approved for clinical use in 2019, has activity against many Gram-negative bacteria. The catechol group of cefiderocol enables it to efficiently enter bacterial cells via the iron/siderophore transport system thereby reducing resistance due to porin channel mutations and efflux pump upregulation. Limited information is reported regarding the binding of cefiderocol to its key proposed target, the transpeptidase penicillin binding protein 3 (PBP3). We report studies on the reaction of cefiderocol and the related cephalosporins ceftazidime and cefepime with Pseudomonas aeruginosa PBP3, including inhibition measurements, protein observed mass spectrometry, and X-ray crystallography. The three cephalosporins form analogous 3-exomethylene products with P. aeruginosa PBP3 following elimination of the C3' side chain. pIC50 and k inact/K i measurements with isolated PBP3 imply ceftazidime and cefiderocol react less efficiently than cefepime and, in particular, meropenem with P. aeruginosa PBP3. Crystal structures inform on conserved and different interactions involved in binding of the three cephalosporins and meropenem to P. aeruginosa PBP3. The results will aid development of cephalosporins with improved PBP3 inhibition properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflicts to declare.
Figures
Similar articles
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria.Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01454-17. doi: 10.1128/AAC.01454-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29061741 Free PMC article.
-
Activity of cefiderocol, imipenem/relebactam, cefepime/taniborbactam and cefepime/zidebactam against ceftolozane/tazobactam- and ceftazidime/avibactam-resistant Pseudomonas aeruginosa.J Antimicrob Chemother. 2022 Sep 30;77(10):2809-2815. doi: 10.1093/jac/dkac241. J Antimicrob Chemother. 2022. PMID: 35904000
-
Burkholderia pseudomallei Clinical Isolates Are Highly Susceptible In Vitro to Cefiderocol, a Siderophore Cephalosporin.Antimicrob Agents Chemother. 2021 Jan 20;65(2):e00685-20. doi: 10.1128/AAC.00685-20. Print 2021 Jan 20. Antimicrob Agents Chemother. 2021. PMID: 33168603 Free PMC article.
-
Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin.Clin Infect Dis. 2019 Nov 13;69(Suppl 7):S538-S543. doi: 10.1093/cid/ciz826. Clin Infect Dis. 2019. PMID: 31724047 Free PMC article. Review.
Cited by
-
Binding assays enable discovery of Tet(X) inhibitors that combat tetracycline destructase resistance.Chem Sci. 2025 May 7;16(22):9691-9704. doi: 10.1039/d5sc00964b. eCollection 2025 Jun 4. Chem Sci. 2025. PMID: 40342919 Free PMC article.
-
Specific variants in ftsI reduce carbapenem susceptibility in Pseudomonas aeruginosa.Microbiol Spectr. 2025 Aug 5;13(8):e0102725. doi: 10.1128/spectrum.01027-25. Epub 2025 Jul 7. Microbiol Spectr. 2025. PMID: 40621918 Free PMC article.
References
-
- Tacconelli E., Magrini N., Carmeli Y., Harbarth S., Kahlmeter G., Kluytmans J., Mendelson M., Pulcini C., Singh N. and Theuretzbacher U., World Health Organisation Global Priority List of Antibiotic Resistant Bacteria to Guide Research, Discovery and Development of New Antibiotics, Geneva, 2017
LinkOut - more resources
Full Text Sources
Miscellaneous

