Association of white matter hyperintensities with cognitive decline and neurodegeneration
- PMID: 39328245
- PMCID: PMC11425965
- DOI: 10.3389/fnagi.2024.1412735
Association of white matter hyperintensities with cognitive decline and neurodegeneration
Abstract
Background: The relationship between white matter hyperintensities (WMH) and the core features of Alzheimer's disease (AD) remains controversial. Further, due to the prevalence of co-pathologies, the precise role of WMH in cognition and neurodegeneration also remains uncertain.
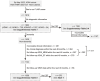
Methods: Herein, we analyzed 1803 participants with available WMH volume data, extracted from the ADNI database, including 756 cognitively normal controls, 783 patients with mild cognitive impairment (MCI), and 264 patients with dementia. Participants were grouped according to cerebrospinal fluid (CSF) pathology (A/T profile) severity. Linear regression analysis was applied to evaluate the factors associated with WMH volume. Modeled by linear mixed-effects, the increase rates (Δ) of the WMH volume, cognition, and typical neurodegenerative markers were assessed. The predictive effectiveness of WMH volume was subsequently tested using Cox regression analysis, and the relationship between WMH/ΔWMH and other indicators such as cognition was explored through linear regression analyses. Furthermore, we explored the interrelationship among amyloid-β deposition, cognition, and WMH using mediation analysis.
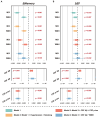
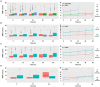
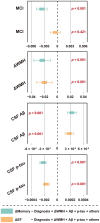
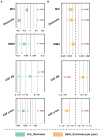
Results: Higher WMH volume was associated with older age, lower CSF amyloid-β levels, hypertension, and smoking history (all p ≤ 0.001), as well as cognitive status (MCI, p < 0.001; dementia, p = 0.008), but not with CSF tau levels. These results were further verified in any clinical stage, except hypertension and smoking history in the dementia stage. Although WMH could not predict dementia conversion, its increased levels at baseline were associated with a worse cognitive performance and a more rapid memory decline. Longitudinal analyses showed that baseline dementia and positive amyloid-β status were associated with a greater accrual of WMH volume, and a higher ΔWMH was also correlated with a faster cognitive decline. In contrast, except entorhinal cortex thickness, the WMH volume was not found to be associated with any other neurodegenerative markers. To a lesser extent, WMH mediates the relationship between amyloid-β and cognition.
Conclusion: WMH are non-specific lesions that are associated with amyloid-β deposition, cognitive status, and a variety of vascular risk factors. Despite evidence indicating only a weak relationship with neurodegeneration, early intervention to reduce WMH lesions remains a high priority for preserving cognitive function in the elderly.
Keywords: Alzheimer’s disease; Aβ; WMH; cerebral small vessel disease; cognition; neurodegeneration.
Copyright © 2024 Li, Li, Xu, Zhong, Wang, Liu and For the Alzheimer’s Disease Neuroimaging Initiative.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
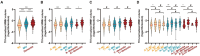
References
-
- Bernal J., Schreiber S., Menze I., Ostendorf A., Pfister M., Geisendörfer J., et al. (2023). Arterial hypertension and Β-amyloid accumulation have spatially overlapping effects on posterior white matter Hyperintensity volume: a cross-sectional study. Alzheimers Res. Ther. 15:97. doi: 10.1186/S13195-023-01243-4, PMID: - DOI - PMC - PubMed
-
- Bittner T., Zetterberg H., Teunissen C. E., Ostlund R. E., Jr., Militello M., Andreasson U., et al. (2016). Technical performance of a novel, fully automated Electrochemiluminescence immunoassay for the quantitation of Β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 12, 517–526. doi: 10.1016/J.Jalz.2015.09.009 - DOI - PubMed
-
- Bos I., Verhey F. R., Ramakers I., Jacobs H., Soininen H., Freund-Levi Y., et al. (2017). Cerebrovascular and amyloid pathology in Predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res. Ther. 9:101. doi: 10.1186/S13195-017-0328-9, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

