Pre-transplantation levels of lysine (K)-specific methyltransferase 2A (KMT2A) partial tandem duplications can predict relapse of acute myeloid leukemia patients following haploidentical donor hematopoietic stem cell transplantation
- PMID: 39328249
- PMCID: PMC11427034
- DOI: 10.1097/BS9.0000000000000207
Pre-transplantation levels of lysine (K)-specific methyltransferase 2A (KMT2A) partial tandem duplications can predict relapse of acute myeloid leukemia patients following haploidentical donor hematopoietic stem cell transplantation
Abstract
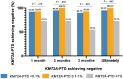
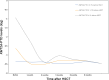
We aimed to identify dynamic changes of lysine (K)-specific methyltransferase 2A partial tandem duplications (KMT2A-PTD) before and after haploidentical donor hematopoietic stem cell transplantation (HID HSCT) and explore the prognostic value of pre-transplantation levels of KMT2A-PTD in acute myeloid leukemia (AML) receiving HID HSCT. Consecutive 64 AML patients with KMT2A-PTD positivity at diagnosis receiving HID HSCT were included in this study. Patients with KMT2A-PTD ≥1% before HSCT had a slower decrease of KMT2A-PTD after HID HSCT. Patients with KMT2A-PTD ≥1% before HID HSCT had a higher cumulative incidence of relapse (36.4%, 95% confidence interval [CI]: 6.3%-66.5%) at 2 years after HSCT than those with KMT2A-PTD <1% (7.5%, 95% CI: 0.3%-14.7%, P = .010). In multivariable analysis, KMT2A-PTD ≥1% before HID HSCT was the only independent risk factor for relapse (hazard ratio [HR]: 4.90; 95% CI: 1.22-19.59; P = .025). Thus, pre-transplantation levels of KMT2A-PTD could predict relapse in AML patients following HID HSCT.
Keywords: Acute myeloid leukemia; Allogeneic hematopoietic stem cell transplantation; Haploidentical; KMT2A-PTD; Relapse.
Copyright © 2024 The Authors. Published by Wolters Kluwer Health Inc., on behalf of the Chinese Medical Association (CMA) and Institute of Hematology, Chinese Academy of Medical Sciences & Peking Union Medical College (IHCAMS).
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Monitoring of post-transplant MLL-PTD as minimal residual disease can predict relapse after allogeneic HSCT in patients with acute myeloid leukemia and myelodysplastic syndrome.BMC Cancer. 2022 Jan 3;22(1):11. doi: 10.1186/s12885-021-09051-5. BMC Cancer. 2022. PMID: 34979982 Free PMC article.
-
Hematopoietic stem cell transplantation for pediatric acute myeloid leukemia patients with KMT2A rearrangement; A nationwide retrospective analysis in Japan.Leuk Res. 2019 Dec;87:106263. doi: 10.1016/j.leukres.2019.106263. Epub 2019 Oct 25. Leuk Res. 2019. PMID: 31707119
-
Minimal residual disease monitoring and preemptive immunotherapies for frequent 11q23 rearranged acute leukemia after allogeneic hematopoietic stem cell transplantation.Ann Hematol. 2021 May;100(5):1267-1281. doi: 10.1007/s00277-021-04488-x. Epub 2021 Mar 13. Ann Hematol. 2021. PMID: 33712867
-
Prognostic significance of KMT2A-PTD in patients with acute myeloid leukaemia: a systematic review and meta-analysis.BMJ Open. 2023 Feb 1;13(2):e062376. doi: 10.1136/bmjopen-2022-062376. BMJ Open. 2023. PMID: 36725100 Free PMC article.
-
Effects of different types of allogeneic hematopoietic stem cell transplantation donors on Philadelphia chromosome-positive acute lymphoblastic leukemia during the tyrosine kinase inhibitor era: A systematic review and meta-analysis.Hematol Oncol Stem Cell Ther. 2023 Apr 4;16(3):197-208. doi: 10.1016/j.hemonc.2021.09.007. Hematol Oncol Stem Cell Ther. 2023. PMID: 34743893
Cited by
-
Mechanism of USP11 regulated SIRT3/ROS in drug resistance of acute myeloid leukemia.Clin Exp Med. 2025 Jul 30;25(1):267. doi: 10.1007/s10238-025-01814-9. Clin Exp Med. 2025. PMID: 40736712 Free PMC article.
-
Increased Co-Expression of PD-L1 and CTLA-4 Predicts Poor Overall Survival in Patients with Acute Myeloid Leukemia Following Allogeneic Hematopoietic Stem Cell Transplantation.Immunotargets Ther. 2025 Jan 20;14:25-33. doi: 10.2147/ITT.S500723. eCollection 2025. Immunotargets Ther. 2025. PMID: 39866547 Free PMC article.
References
-
- Lv M, Gorin NC, Huang XJ. A vision for the future of allogeneic hematopoietic stem cell transplantation in the next decade. Sci Bull (Beijing) 2022;67(19):1921–1924. - PubMed
-
- Cao Y, Zhang C, Cao L, Mo X, Hu X. Quizartinib is a good option for AML patients with FLT3-ITD mutations. Innov Med 2023;1(1):100007.
-
- Deng D, Shen M, Zhang X, et al. . Basiliximab is the potential solution for severe liver chronic GVHD: a prospective pilot study. Innov Med 2023;1(1):100009. doi:10.59717/j.xinn-med.2023.100009.
LinkOut - more resources
Full Text Sources
