Exploring the potential of the convergence between extracellular vesicles and CAR technology as a novel immunotherapy approach
- PMID: 39328262
- PMCID: PMC11424882
- DOI: 10.1002/jex2.70011
Exploring the potential of the convergence between extracellular vesicles and CAR technology as a novel immunotherapy approach
Abstract
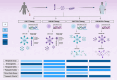
Cancer therapy is a dynamically evolving field, witnessing the emergence of innovative approaches that offer a promising outlook for patients grappling with persistent disease. Within the realm of therapeutic exploration, chimeric antigen receptor (CAR) T cells as well as CAR NK cells, have surfaced as novel approaches, each possessing unique attributes and transformative potential. Immune cells engineered to express CARs recognizing tumour-specific antigens, have shown remarkable promise in treating terminal cancers by combining the precision of antibody specificity with the potent cytotoxic function of T cells. However, their application in solid tumours is still in its nascent stages, presenting unique major challenges. On the same note, CAR NK cells offer a distinct immunotherapeutic approach, utilizing CARs on NK cells, providing advantages in safety, manufacturing simplicity, and a broader scope for cancer treatment. Extracellular vesicles (EVs) have emerged as promising therapeutic agents due to their ability to carry crucial biomarkers and biologically active molecules, serving as vital messengers in the intercellular communication network. In the context of cancer, the therapeutic potential of EVs lies in delivering tumour-suppressing proteins, nucleic acid components, or targeting drugs with precision, thereby redefining the paradigm of precision medicine. The fusion of CAR technology with the capabilities of EVs has given rise to a new therapeutic frontier. CAR T EVs and CAR NK EVs, leveraging the power of EVs, have the potential to alleviate challenges associated with live-cell therapies. EVs are suggested to reduce the side effects linked to CAR T cell therapy and hold the potential to revolutionize the penetrance in solid tumours. EVs act as carriers of pro-apoptotic molecules and RNA components, enhancing immune responses and thereby expanding their therapeutic potential. In this review article, we navigate dynamic landscapes, with our objective being to evaluate comparative efficacy, safety profiles, manufacturing complexities, and clinical applicability.
Keywords: NK cells; T cells; chimeric antigen receptors; extracellular vesicles; immunotherapy.
© 2024 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Achar, S. R. , Bourassa, F. X. P. , Rademaker, T. J. , Lee, A. , Kondo, T. , Salazar‐Cavazos, E. , Davies, J. S. , Taylor, N. , François, P. , & Altan‐Bonnet, G. (2022). Universal antigen encoding of T cell activation from high‐dimensional cytokine dynamics. Science (New York, N.Y.), 376(6595), 880–884. 10.1126/science.abl5311 - DOI - PubMed
-
- Alabanza, L. , Pegues, M. , Geldres, C. , Shi, V. , Wiltzius, J. J. W. , Sievers, S. A. , Yang, S. , & Kochenderfer, J. N. (2017). Function of novel anti‐CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Molecular Therapy: The Journal of the American Society of Gene Therapy, 25(11), 2452–2465. 10.1016/j.ymthe.2017.07.013 - DOI - PMC - PubMed
-
- Alizadeh, D. , Wong, R. A. , Gholamin, S. , Maker, M. , Aftabizadeh, M. , Yang, X. , Pecoraro, J. R. , Jeppson, J. D. , Wang, D. , Aguilar, B. , Starr, R. , Larmonier, C. B. , Larmonier, N. , Chen, M. H. , Wu, X. , Ribas, A. , Badie, B. , Forman, S. J. , & Brown, C. E. (2021). IFNγ is critical for CAR T cell‐mediated myeloid activation and induction of endogenous immunity. Cancer Discovery, 11(9), 2248–2265. 10.1158/2159-8290.CD-20-1661 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
