A molecular standard for circulating HBV RNA detection and quantification assays in patients with chronic hepatitis B
- PMID: 39328324
- PMCID: PMC11424956
- DOI: 10.1016/j.jhepr.2024.101124
A molecular standard for circulating HBV RNA detection and quantification assays in patients with chronic hepatitis B
Abstract
Background & aims: Circulating HBV RNAs have been proposed as a biomarker that reflects the transcriptional activity of covalently closed circular DNA (cccDNA) and may help to evaluate HBV treatment activity. Different research assays have been proposed and, although two PCR-based research use only investigational assays have been developed, the lack of standardized protocols represents an important limitation. Here we have designed and generated a stable clonal cell line producing an RNA-based standard for the calibration of PCR-based circulating HBV RNA assays.
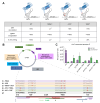
Methods: HBV RNA-producing Huh7-derived stable cell lines were generated by transfecting pTriEX plasmids containing 1.1 unit length HBV DNA genomes carrying mutations in the catalytic site (YMAA mutation) and the TP domain (Y63F) of the polymerase, and the ε-loop of the pregenomic (pg)RNA (mutation A1G).
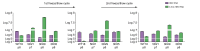
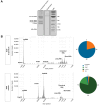
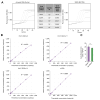
Results: The clonal cell line (Huh7-3D29), carrying a double YMAA and Y63F mutation, displayed, and maintained over several passages in culture, a high RNA secretion phenotype with negligible residual secreted HBV DNA. Density gradient centrifugation showed that most of the secreted HBV RNA from Huh7-3D29 cells was detected in naked capsid and virion-like particles and only a minority in small extracellular vescicles. Nanopore sequencing of 5'RACE products shows that the majority of the Huh7-3D29-secreted HBV RNAs start at the 5' end of pgRNA and pgRNA-derived spliced RNAs. Finally, Huh7-3D29 cells showed a high and up-scalable secreted RNA yield allowing 1,300 standard curves in 9 days from one flask.
Conclusion: We generated a clonal cell line that produces high quantities of HBV RNAs with very low quantities of contaminating HBV DNAs, representing a stable source of RNA standard for HBV RNA assay calibration.
Impact and implications: Several investigational assays and two research use only assays have been developed to detect and quantify circulating HBV RNAs, an emerging biomarker of covalently closed circular DNA transcriptional activity and target engagement by new HBV treatments. The lack of a unique molecular standard for circulating HBV RNA quantification represents an important limitation. Here we describe the generation of a stable clonal cell line producing and secreting an RNA-based standard containing all the HBV RNA species found in HBV patients' sera (e.g. pgRNA, HBx transcripts). This new RNA standard can be used to calibrate all PCR-based assays for circulating HBV RNA quantification to evaluate, in a non-invasive manner, the size of the transcriptionally active cccDNA pool and the activity of novel strategies aimed at curing HBV infection.
Keywords: biomarker; chronic hepatitis B (CHB); hepatitis B virus (HBV); pregenomic RNA (pgRNA); standard.
© 2024 The Authors.
Figures


References
-
- Global hepatitis report. 2017. https://www.who.int/publications-detail-redirect/global-hepatitis-report...
-
- Ghany M.G., Buti M., Lampertico P., et al. 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: report from the 2022 AASLD-EASL HBV/HDV treatment endpoints conference. J Hepatol. 2023 doi: 10.1016/j.jhep.2023.06.002. S0168-8278(23)00417-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

