Peripheral NK cell phenotypic alteration and dysfunctional state post hepatitis B subviral particles stimulation in CHB patients: evading immune surveillance
- PMID: 39328404
- PMCID: PMC11424423
- DOI: 10.3389/fimmu.2024.1427519
Peripheral NK cell phenotypic alteration and dysfunctional state post hepatitis B subviral particles stimulation in CHB patients: evading immune surveillance
Abstract
Background: The relationship between chronic hepatitis B (CHB) infection and natural killer (NK) cell dysfunction is well-established, but the specific role of HBV viral antigens in driving NK cell impairment in patients with CHB remains unclear. This study investigates the modulatory effects of hepatitis B virus subviral particles (HBVsvp, a representative model for HBsAg) on the phenotypic regulation (activating and inhibitory receptors), cytokine production and cytotoxic potential of peripheral blood mononuclear cell-derived natural killer cells (PBMCs-derived NK cell), which contributes to NK cell dysfunction in CHB infection, potentially serving as an effective HBV immune evasion strategy by the virus.
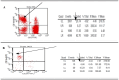
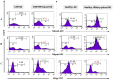
Methods: NK cells were isolated from peripheral blood of patients with CHB (n=5) and healthy individuals (n=5), stimulated with HBVsvp. Subsequent flow cytometric characterization involved assessing changes in activating (NKp46 and NKG2D) and inhibitory (CD94) receptors expression, quantifying TNF-α and IFN- γ cytokine secretion, and evaluating the cytotoxic response against HepG2.2.15 cells with subsequent HBVsvp quantification.
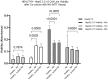
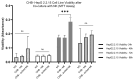
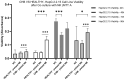
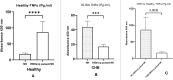
Results: In CHB patients, in vitro exposure of PBMCs-derived NK cell with HBVsvp (represent HBsAg model) significantly reduced NK cell-activating receptors expression (P = 0.022), increased expression of CD94 + NK cells (p = 0.029), accompanied with a reduced TNF-α - IFN-γ cytokine levels, and impaired cytotoxic capacity (evidenced by increased cell proliferation and elevated HBVsvp levels in co-cultures with HepG2.2.15 cells in a time-dependent), relative to healthy donors.
Conclusion: These findings suggest that HBVsvp may induce dysfunctional NK cell responses characterized by phenotypic imbalance with subsequent reduction in cytokine and cytotoxic levels, indicating HBVsvp immunosuppressive effect that compromises antiviral defense in CHB patients. These data enhance our understanding of NK cell interactions with HBsAg and highlight the potential for targeting CD94 inhibitory receptors to restore NK cell function as an immunotherapeutic approach. Further clinical research is needed to validate these observations and establish their utility as reliable biomarkers.
Keywords: CD94 inhibitory receptor; HB subviral particles (HBVsvp); HbsAg; NKp46 and NKG2D activating receptors; chronic hepatitis B (CHB); cytokine; immunotherapy; natural killer (NK) cells.
Copyright © 2024 Selim, Suef, Saied, Abdel-Maksoud, Almutairi, Aufy, Mousa, Mansour and Farag.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
HBsAg stimulates NKG2D receptor expression on natural killer cells and inhibits hepatitis C virus replication.Hepatobiliary Pancreat Dis Int. 2018 Jun;17(3):233-240. doi: 10.1016/j.hbpd.2018.03.010. Epub 2018 Mar 26. Hepatobiliary Pancreat Dis Int. 2018. PMID: 29625837
-
Natural Killer p46 Controls Hepatitis B Virus Replication and Modulates Liver Inflammation.PLoS One. 2015 Aug 20;10(8):e0135874. doi: 10.1371/journal.pone.0135874. eCollection 2015. PLoS One. 2015. PMID: 26291078 Free PMC article.
-
Frequency and role of NKp46 and NKG2A in hepatitis B virus infection.PLoS One. 2017 Mar 22;12(3):e0174103. doi: 10.1371/journal.pone.0174103. eCollection 2017. PLoS One. 2017. PMID: 28328926 Free PMC article.
-
Endocytosis as a mechanism of regulating natural killer cell function: unique endocytic and trafficking pathway for CD94/NKG2A.Immunol Res. 2009;43(1-3):210-22. doi: 10.1007/s12026-008-8072-7. Immunol Res. 2009. PMID: 18979076 Free PMC article. Review.
-
Investigation of NK cell function and their modulation in different malignancies.Immunol Res. 2012 Apr;52(1-2):139-56. doi: 10.1007/s12026-012-8285-7. Immunol Res. 2012. PMID: 22442005 Review.
Cited by
-
Therapeutic interventions aimed at cccDNA: unveiling mechanisms and evaluating the potency of natural products.Front Cell Infect Microbiol. 2025 Jun 17;15:1598872. doi: 10.3389/fcimb.2025.1598872. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40599653 Free PMC article. Review.
References
-
- Organization, W.H . Consultation on the global health sector strategies on HIV, viral hepatitis and sexually transmitted infections (STIs), 2022–2030: virtual meeting report: Copenhagen, Denmark and online 16–17 June 2021. Geneva, Switzerland: World Health Organization Regional Office for Europe; (2022).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

