Hydride Shuttle Catalysis: From Conventional to Inverse Mode
- PMID: 39328743
- PMCID: PMC11423322
- DOI: 10.1021/jacsau.4c00532
Hydride Shuttle Catalysis: From Conventional to Inverse Mode
Abstract
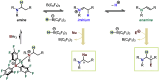
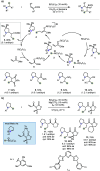
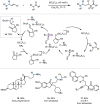
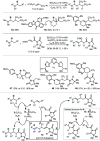
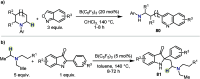
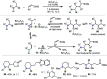
Hydride shuttle catalysis has emerged as a powerful synthetic platform, enabling the selective formation of C-C bonds to yield sp3-rich structures. By virtue of the compelling reactivity of sterically encumbered Lewis acids from the frustrated Lewis pair regime, hydride shuttle catalysis enables the regioselective functionalization of alkyl amines at either the α- or β-position. In contrast to classical Lewis acid reactivity, the increased steric hindrance prevents interaction with the Lewis basic amine itself, instead leading to reversible abstraction of a hydride from the amine α-carbon. The created positive charge facilitates the occurrence of transformations before hydride rebound or a similar capture event happen. In this Perspective, we outline a broad selection of transformations featuring hydride shuttle catalysis, as well as the recently developed approach of inverse hydride shuttle catalysis. Both strategies give rise to a wide array of functionalized amines and offer elegant approaches to otherwise elusive bond formations.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















Similar articles
-
Hydride-Abstraction-Initiated Catalytic Stereoselective Intermolecular Bond-Forming Processes.Acc Chem Res. 2022 Dec 6;55(23):3537-3550. doi: 10.1021/acs.accounts.2c00638. Epub 2022 Nov 17. Acc Chem Res. 2022. PMID: 36384272
-
Lewis Acid-Driven Inverse Hydride Shuttle Catalysis.Angew Chem Int Ed Engl. 2024 Jul 1;63(27):e202320001. doi: 10.1002/anie.202320001. Epub 2024 May 29. Angew Chem Int Ed Engl. 2024. PMID: 38551113
-
Rh-Catalyzed Intermolecular Reactions of α-Alkyl-α-Diazo Carbonyl Compounds with Selectivity over β-Hydride Migration.Acc Chem Res. 2016 Jan 19;49(1):115-27. doi: 10.1021/acs.accounts.5b00425. Epub 2015 Dec 21. Acc Chem Res. 2016. PMID: 26689221 Free PMC article.
-
Electronic and Steric Tuning of a Prototypical Piano Stool Complex: Rh(III) Catalysis for C-H Functionalization.Acc Chem Res. 2018 Jan 16;51(1):170-180. doi: 10.1021/acs.accounts.7b00444. Epub 2017 Dec 22. Acc Chem Res. 2018. PMID: 29272106 Free PMC article. Review.
-
The azomethine ylide route to amine C-H functionalization: redox-versions of classic reactions and a pathway to new transformations.Acc Chem Res. 2015 Feb 17;48(2):317-28. doi: 10.1021/ar5003768. Epub 2015 Jan 6. Acc Chem Res. 2015. PMID: 25560649 Free PMC article. Review.
Cited by
-
Modular alkyl growth in amines via the selective insertion of alkynes into C-C bonds.Nat Chem. 2025 Sep;17(9):1323-1330. doi: 10.1038/s41557-025-01849-1. Epub 2025 Jun 12. Nat Chem. 2025. PMID: 40506572
References
-
- Lewis G. N.; Murrell J. N. BOOKS: Valence and the Structure of Atoms and Molecules. Phys. Teach. 1968, 6 (1), 46–47. 10.1119/1.2352399. - DOI
-
- Brown H. C.; Schlesinger H. I.; Cardon S. Z. Studies in Stereochemistry. I. Steric Strains as a Factor in the Relative Stability of Some Coördination Compounds of Boron. J. Am. Chem. Soc. 1942, 64 (2), 325–329. 10.1021/ja01254a031. - DOI
Publication types
LinkOut - more resources
Full Text Sources
