Isolated Pt Atoms Stabilized by Ga2O3 Clusters Confined in ZSM-5 for Nonoxidative Activation of Ethane
- PMID: 39328764
- PMCID: PMC11423304
- DOI: 10.1021/jacsau.4c00480
Isolated Pt Atoms Stabilized by Ga2O3 Clusters Confined in ZSM-5 for Nonoxidative Activation of Ethane
Abstract
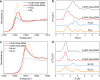
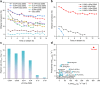
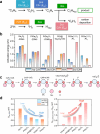
Selective activation of light alkanes is an essential reaction in the petrochemical industry for producing commodity chemicals, such as light olefins and aromatics. Because of the much higher intrinsic activities of noble metals in comparison to non-noble metals, it is desirable to employ solid catalysts with low noble metal loadings to reduce the cost of catalysts. Herein, we report the introduction of a tiny amount of Pt (at levels of hundreds of ppm) as a promoter of the Ga2O3 clusters encapsulated in ZSM-5 zeolite, which leads to ∼20-fold improvement in the activity for ethane dehydrogenation reaction. A combination of experimental and theoretical studies shows that the isolated Pt atoms stabilized by small Ga2O3 clusters are the active sites for activating the inert C-H bonds in ethane. The synergy of atomically dispersed Pt and Ga2O3 clusters confined in the 10MR channels of ZSM-5 can serve as a bifunctional catalyst for the direct ethane-benzene coupling reaction for the production of ethylbenzene, surpassing the performances of the counterpart catalysts made with PtGa nanoclusters and nanoparticles.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Li X.; Pei C.; Gong J. Shale gas revolution: Catalytic conversion of C1–C3 light alkanes to value-added chemicals. Chem. 2021, 7 (7), 1755–1801. 10.1016/j.chempr.2021.02.002. - DOI
-
- Xiang Y.; Wang H.; Cheng J.; Matsubu J. Progress and prospects in catalytic ethane aromatization. Catal. Sci. Technol. 2018, 8 (6), 1500–1516. 10.1039/C7CY01878A. - DOI
-
- Ren T.; Patel M.; Blok K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31 (4), 425–451. 10.1016/j.energy.2005.04.001. - DOI
-
- Yacob S.; Caulfield M.; Larson R. B.; Gomez E.; Meyer R. J. The Interplay between Process Conceptualization and Experimental Research—Accelerating and Guiding Catalysis to Process Breakthroughs. ACS Catal. 2022, 12 (17), 10621–10628. 10.1021/acscatal.2c02116. - DOI
LinkOut - more resources
Full Text Sources
