Screening key sorghum germplasms for low-nitrogen tolerance at the seedling stage and identifying from the carbon and nitrogen metabolism
- PMID: 39328797
- PMCID: PMC11424420
- DOI: 10.3389/fpls.2024.1340509
Screening key sorghum germplasms for low-nitrogen tolerance at the seedling stage and identifying from the carbon and nitrogen metabolism
Abstract
Introduction: Sorghum (Sorghum bicolor L.) can withstand drought and heat stress and efficiently utilize water and nutrients. However, the underlying mechanism of its tolerance to low-nitrogen (N) stress remains poorly understood.
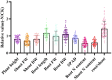
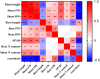
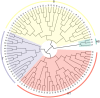
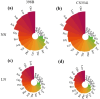
Materials and methods: This study assessed low-N tolerance in 100 sorghum-inbred lines and identified those with exceptional resilience. Principal component analysis, Pearson's correlation, and Y value analysis were used to examine various seedling growth metrics, including plant and root dimensions, biomass, chlorophyll content, root N content, shoot N content, and root/shoot ratio.
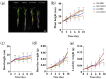
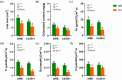
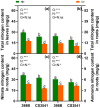
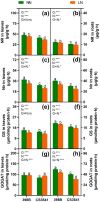
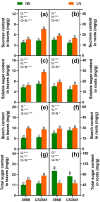
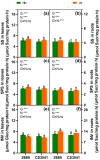
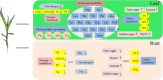
Results and discussion: The genotypes were categorized into four distinct groups based on their respective Y values, revealing a spectrum from highly tolerant to sensitive. Low-N-tolerant sorghum lines maintained higher photosynthetic rates and exhibited increased enzymatic activities linked to carbon and N metabolism in the leaves and roots. Furthermore, low-N-tolerant genotypes had higher levels of key amino acids, including cystine, glycine, histidine, isoleucine, leucine, phenylalanine, threonine, and tyrosine, indicating a robust internal metabolic response to N deficiency.
Conclusion: This study provides a comprehensive and reliable approach for the evaluation of sorghum tolerance to low-N environments, sheds light on its morphological and physiological adaptations, and provides valuable insights for future breeding programs and agricultural practices.
Keywords: C metabolism; N metabolism; Sorghum bicolor; genotypic variation; low-N tolerance screening; phenotype.
Copyright © 2024 Liu, Gu, Li, Feng, Liu, Shi and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Tryptophan regulates sorghum root growth and enhances low nitrogen tolerance.Plant Physiol Biochem. 2024 Jul;212:108737. doi: 10.1016/j.plaphy.2024.108737. Epub 2024 May 16. Plant Physiol Biochem. 2024. PMID: 38763003
-
Evidence for the involvement of hydraulic root or shoot adjustments as mechanisms underlying water deficit tolerance in two Sorghum bicolor genotypes.J Plant Physiol. 2016 Mar 15;192:13-20. doi: 10.1016/j.jplph.2016.01.002. Epub 2016 Jan 8. J Plant Physiol. 2016. PMID: 26803215
-
Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling.Plant Physiol Biochem. 2020 Jun;151:233-242. doi: 10.1016/j.plaphy.2020.03.027. Epub 2020 Mar 23. Plant Physiol Biochem. 2020. PMID: 32234662
-
Drought and High Temperature Stress in Sorghum: Physiological, Genetic, and Molecular Insights and Breeding Approaches.Int J Mol Sci. 2021 Sep 11;22(18):9826. doi: 10.3390/ijms22189826. Int J Mol Sci. 2021. PMID: 34575989 Free PMC article. Review.
-
Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum.Int J Mol Sci. 2024 Jun 12;25(12):6464. doi: 10.3390/ijms25126464. Int J Mol Sci. 2024. PMID: 38928168 Free PMC article. Review.
References
-
- Addy S., Jr-Niedziela C. E., Reddy M. P. (2010). Effect of nitrogen fertilization on stay-green and senescent sorghum hybrids in sand culture. J. Plant Nutr. 33, 185–199. doi: 10.1080/01904160903434253 - DOI
-
- Carcedo A. J., Mayor L., Demarco P., Morris G. P., Lingenfelser J., Messina C. D., et al. . (2022). Environment characterization in Sorghum (Sorghum bicolor L.) by modeling water-deficit and heat patterns in the Great Plains Region, United States. Front. Plant Sci. 13, 768610. doi: 10.3389/fpls.2022.768610 - DOI - PMC - PubMed
-
- Du C., Zhang M., Zhou X., Bai Y., Wang L., Zhang L., et al. . (2023). Revealing the relationship between nitrogen use efficiency-related QTLs and carbon and nitrogen metabolism regulation in poplar. GCB. Bioenergy. 15, 575–592. doi: 10.1111/gcbb.13040 - DOI
LinkOut - more resources
Full Text Sources

