Safety and Tolerability of Suprachoroidal Axitinib Injectable Suspension, for Neovascular Age-related Macular Degeneration; Phase I/IIa Open-Label, Dose-Escalation Trial
- PMID: 39328827
- PMCID: PMC11426123
- DOI: 10.1016/j.xops.2024.100586
Safety and Tolerability of Suprachoroidal Axitinib Injectable Suspension, for Neovascular Age-related Macular Degeneration; Phase I/IIa Open-Label, Dose-Escalation Trial
Abstract
Purpose: To evaluate the safety and tolerability of a single dose of axitinib injectable suspension (CLS-AX), a pan-anti-VEGF tyrosine kinase inhibitor (TKI), administered via suprachoroidal injection in patients with neovascular age-related macular degeneration (nAMD).
Design: Phase I/IIa, open-label, sequential dose escalation.
Participants: Anti-VEGF treatment-experienced patients with active subfoveal choroidal neovascularization secondary to nAMD.
Methods: The study included 4 cohorts (0.03, 0.10, 0.50, and 1.0 mg) of approximately 5 patients each enrolled in a dose-escalating fashion. Enrolled patients received intravitreal aflibercept (2 mg) followed by a single unilateral dose of CLS-AX 1 month later. All patients were followed monthly for 3 months with the option of an additional 3 months of extended follow-up for cohorts 2 to 4. End points included systemic and ocular safety and tolerability, visual acuity, retinal thickness, and need for aflibercept therapy.
Main outcome measures: The number of patients reporting treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs), changes in ophthalmic examinations, and the number of patients qualifying for additional therapy for nAMD based on protocol-defined criteria.
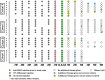
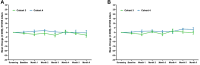
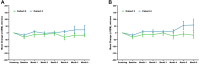
Results: OASIS enrolled 27 patients with nAMD with mean age of 81 years, mean duration of nAMD diagnosis of 54 months, and between 5 and 90 prior anti-VEGF treatments. Twenty-six patients completed through 3 months, with 14 entering and completing the 3-month extension. No SAEs, drug-related TEAEs, or TEAEs leading to discontinuation were observed after CLS-AX administration; there were no adverse events related to ocular inflammation, vasculitis, intraocular pressure, or dispersion of drug into the vitreous or anterior chamber. Through 6 months, stable mean best-corrected visual acuity and stable mean central subfield thickness (CST) were observed, suggestive of TKI biologic effect. No aflibercept therapy was administered up to 3 months in 58% (15/26) of patients who completed 3 months of follow-up in OASIS. In the Extension, 57% (8/14) of patients went up to 6 months without receiving aflibercept therapy.
Conclusions: Up to 1.0 mg CLS-AX, a highly potent TKI targeted to the suprachoroidal space (SCS) via the SCS Microinjector, was well tolerated, with stable mean visual acuity and mean CST. A majority of patients followed for 6 months did not require aflibercept therapy.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: AMD; Age-related macular degeneration; Axitinib; Suprachoroidal; Tyrosine kinase inhibitor.
© 2024 by the American Academy of Ophthalmology.
Figures

References
-
- Friedman D.S., O’Colmain B.J., Muñoz B., et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. - PubMed
-
- Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. - PubMed
-
- Ambati J., Ambati B.K., Yoo S.H., et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. - PubMed
-
- Flaxel C.J., Adelman R.A., Bailey S.T., et al. Age-related macular degeneration Preferred Practice Pattern. Ophthalmology. 2020;127:P1–P65. - PubMed
-
- Singer M.A., Awh C.C., Sadda S., et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–1183. - PubMed
LinkOut - more resources
Full Text Sources

