Recent advances in the synthesis of highly substituted imidazolidines
- PMID: 39328874
- PMCID: PMC11426194
- DOI: 10.1039/d4ra06010e
Recent advances in the synthesis of highly substituted imidazolidines
Abstract
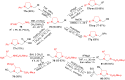
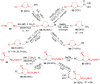
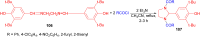
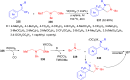
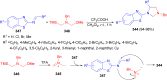
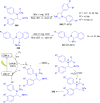
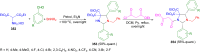
Imidazolidine is a saturated heterocycle with a cyclic aminal core that can be found in natural products and biologically active molecules. Additionally, these heterocyclic compounds have been utilized as chiral ligands, N-heterocyclic carbene precursors, and catalysts in organic synthesis. This review is an attempt to compile the literature of various synthetic procedures of highly substituted imidazolidines, chiral imidazolidines with high diastereoselectivities and enantioselectivities, bis-imidazolidines, and spiro-imidazolidines, as well as their pharmacological properties during the period from 1949 to 2023.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
We have no conflicts of interest to disclose.
Figures

















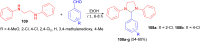




















































Similar articles
-
Pharmaceutical importance and synthetic strategies for imidazolidine-2-thione and imidazole-2-thione derivatives.Pak J Biol Sci. 2011 Dec 15;14(24):1076-89. doi: 10.3923/pjbs.2011.1076.1089. Pak J Biol Sci. 2011. PMID: 22335047
-
Enantioselective [3+2] Cycloaddition of Donor-Acceptor Aziridines and Imines to Construct 2,5-trans-Imidazolidines.Chemistry. 2023 Mar 28;29(18):e202203757. doi: 10.1002/chem.202203757. Epub 2023 Feb 24. Chemistry. 2023. PMID: 36602265
-
Transition-metal-catalyzed enantioselective heteroatom-hydrogen bond insertion reactions.Acc Chem Res. 2012 Aug 21;45(8):1365-77. doi: 10.1021/ar300051u. Epub 2012 May 31. Acc Chem Res. 2012. PMID: 22651217
-
Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals.Molecules. 2021 Jan 28;26(3):677. doi: 10.3390/molecules26030677. Molecules. 2021. PMID: 33525514 Free PMC article. Review.
-
Malononitrile dimer as a privileged reactant in design and skeletal diverse synthesis of heterocyclic motifs.Mol Divers. 2018 Feb;22(1):207-224. doi: 10.1007/s11030-017-9807-y. Epub 2018 Jan 3. Mol Divers. 2018. PMID: 29299856 Review.
References
-
- Jiao R. H. Xu S. Liu J. Y. Ge H. M. Ding H. Xu C. Zhu H. L. Tan R. X. Org. Lett. 2006;8:5709–5712. - PubMed
-
- Malgesini B. Forte B. Borghi D. Quartieri F. Gennari C. Papeo G. Chem.–Eur. J. 2009;15:7922–7929. - PubMed
-
- Nakao Y. Kuo J. Yoshida W. Y. Kelly M. Scheuer P. J. Org. Lett. 2003;5:1387–1390. - PubMed
-
- Mao Z. Y. Geng H. Zhang T. T. Ruan Y. P. Ye J. L. Huang P. Q. Org. Chem. Front. 2016;3:24–37.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

