A Randomized, Multicenter Phase 3 Clinical Trial Evaluating Intracanalicular Dexamethasone Insert for the Treatment of Allergic Conjunctivitis
- PMID: 39328900
- PMCID: PMC11424686
- DOI: 10.2147/OPTH.S476419
A Randomized, Multicenter Phase 3 Clinical Trial Evaluating Intracanalicular Dexamethasone Insert for the Treatment of Allergic Conjunctivitis
Abstract
Purpose: To evaluate the efficacy and safety of a dexamethasone intracanalicular insert (DEX) for treatment of allergic conjunctivitis (AC).
Patients and methods: In this multicenter, randomized, double-masked, placebo-controlled phase 3 study, adults (≥18 years) with AC were randomized 1:1 to DEX or placebo insert (PBO) placed bilaterally. Subjects underwent repetitive conjunctival allergen challenges (CAC) across 30 days and were assessed for changes in AC signs and symptoms. The primary endpoint was ocular itching score at 3, 5, and 7 minutes post-CAC at Day 8 (7 days post-insertion). This trial is registered on ClinicalTrials.gov (NCT04050865).
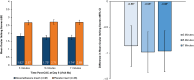
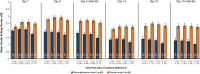
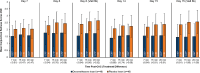
Results: Ninety-six subjects were randomized (n=48 DEX, n=48 PBO). Compared to PBO, there were statistically significant treatment differences favoring DEX for the primary endpoint of mean ocular itching score at Day 8 (-0.86, -0.98, -0.96 at 3, 5, and 7 minutes post-CAC respectively; P<0.0001 for all). Treatment differences favored DEX for all 24 time points across 6 visits and were statistically significant (P<0.05) except for the first post-insertion (Day 7, 3 minutes). For the 18 time points at which conjunctival redness was assessed, DEX had lower scores than PBO (P<0.05 for all). The most common ocular adverse events (AEs) in DEX subjects were eye discharge and irritation. No serious AEs, elevated intraocular pressure, dacryocanaliculitis, or use of rescue medications were reported.
Conclusion: Results of this study support the potential use of dexamethasone insert as a physician-administered, preservative-free treatment for AC, with significant improvements in ocular itching and conjunctival redness compared with placebo. The dexamethasone insert was generally safe with a favorable safety profile.
Keywords: allergy; conjunctivitis; dexamethasone; insert; intracanalicular.
© 2024 Kenyon et al.
Conflict of interest statement
Kenneth Kenyon, Eugene McLaurin, Steven Silverstein, John Meyer, Erik Anderson, and Ravi Patel were study investigators and have no conflicts of interest to disclose for this work. Paul Gomes is an employee of Ora, Inc. Erin Reilly, Srilatha Vantipalli, and Matthew Cheung are employees of Ocular Therapeutix, Inc. Michael H. Goldstein is a former employee of Ocular Therapeutix, Inc. The authors report no other conflicts of interest in this work.
Figures

References
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

