DDI2 protease controls embryonic development and inflammation via TCF11/NRF1
- PMID: 39328932
- PMCID: PMC11424978
- DOI: 10.1016/j.isci.2024.110893
DDI2 protease controls embryonic development and inflammation via TCF11/NRF1
Abstract
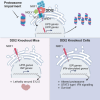
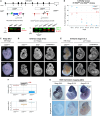
DDI2 is an aspartic protease that cleaves polyubiquitinated substrates. Upon proteotoxic stress, DDI2 activates the transcription factor TCF11/NRF1 (NFE2L1), crucial for maintaining proteostasis in mammalian cells, enabling the expression of rescue factors, including proteasome subunits. Here, we describe the consequences of DDI2 ablation in vivo and in cells. DDI2 knock-out (KO) in mice caused embryonic lethality at E12.5 with severe developmental failure. Molecular characterization of embryos showed insufficient proteasome expression with proteotoxic stress, accumulation of high molecular weight ubiquitin conjugates and induction of the unfolded protein response (UPR) and cell death pathways. In DDI2 surrogate KO cells, proteotoxic stress activated the integrated stress response (ISR) and induced a type I interferon (IFN) signature and IFN-induced proliferative signaling, possibly ensuring survival. These results indicate an important role for DDI2 in the cell-tissue proteostasis network and in maintaining a balanced immune response.
Keywords: Biological sciences; Developmental biology; Immune respons.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

