Fractional Exhaled Nitric Oxide Testing for the Diagnosis and Management of Asthma: a Health Technology Assessment
- PMID: 39329005
- PMCID: PMC11423898
Fractional Exhaled Nitric Oxide Testing for the Diagnosis and Management of Asthma: a Health Technology Assessment
Abstract
Background: Asthma is a common respiratory disease characterized by airflow obstruction caused by inflammation and narrowing of the airways. Nitric oxide is a gas that is present at low levels in the lungs, but that is elevated in the presence of airway inflammation. Fractional exhaled nitric oxide (FeNO) testing may help in the diagnosis and management of asthma by measuring the amount of nitric oxide in the breath. We conducted a health technology assessment of FeNO testing for the diagnosis and management of asthma in children and adults, which included an evaluation of the accuracy, effectiveness, cost-effectiveness, the budget impact of publicly funding FeNO testing, and patient preferences and values.
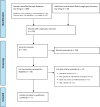
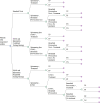
Methods: We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Quality Assessment of Diagnostic Accuracy Studies tool, version 2 (QUADAS-2) and of each systematic review using the Risk of Bias Assessment Tool for Systematic Reviews (ROBIS). We evaluated the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search and conducted cost-utility analyses with a 20-year time horizon from a public payer perspective. We also analyzed the budget impact of publicly funding FeNO testing in children and adults in Ontario. To contextualize the potential value of FeNO testing, we spoke with people with asthma and their care partners.
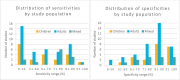
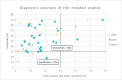
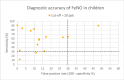
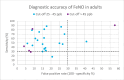
Results: We included 48 primary studies assessing the diagnostic accuracy of FeNO testing and 2 reviews evaluating the effectiveness of FeNO testing for asthma management in the clinical evidence review. The use of FeNO testing for the diagnosis of asthma reported variable (~30% to 90%) sensitivities (GRADE: Very low) and consistently high (~70% to 100%) specificities (GRADE: Low) in children and adults. FeNO testing for asthma management likely reduced exacerbations in children (GRADE: Moderate) and adults (GRADE: Moderate), lowered oral corticosteroid use in children (GRADE: Moderate), and slightly improved lung function in a mixed population (GRADE: Moderate), but little to no improvement was seen in other outcomes. We found that, for asthma diagnosis, FeNO testing in addition to standard testing is cost-effective in children, with an incremental cost-effectiveness ratio (ICER) of $6,192 per quality-adjusted life-year (QALY) gained. FeNO testing is not cost-effective for asthma diagnosis in adults except when a higher FeNO cut-off is applied. For asthma management, the ICER of FeNO testing compared with standard care alone is $103,893 per QALY gained in children and $200,135 per QALY gained in adults. Publicly funding FeNO testing as an adjunct to standard testing for asthma diagnosis over the next 5 years would cost about $0.10 million to $0.22 million for children and $1.19 million to $1.61 million for adults over the next 5 years, and for asthma management would cost about $22.37 million for children and $195.99 million for adults over the next 5 years. Participants were unaware if they had experience with FeNO testing because of its similarity to other types of asthma testing, but they reported valuing the potential of FeNO testing to provide more information about their condition as well as aid in the diagnosis and management. Barriers to access include lack of awareness and the limited availability of FeNO testing across the province.
Conclusions: We found that FeNO testing had good diagnostic specificity (i.e., low false positive rate), supporting its use as an adjunct to standard testing to help rule-in an asthma diagnosis in both children and adults. FeNO testing to monitor and manage asthma likely resulted in a reduction in the number of people who experienced exacerbations and used oral corticosteroids, but may make little to no difference in improving other health outcomes. FeNO testing is likely cost-effective as an additional test to support the diagnosis of asthma in children, as well as in adults when a higher FeNO cut-off is applied, but is likely not cost-effective as an additional test to monitor and manage asthma in both children and adults. We estimate that publicly funding FeNO testing as an adjunct to standard testing for asthma diagnosis in Ontario would result in additional costs of $0.10 million to $0.22 million for children and $1.19 million to $1.61 million for adults over the next 5 years. For monitoring and managing asthma, FeNO testing would result in additional costs of $22.37 million for children and $195.99 million for adults over the next 5 years. People we spoke with were unaware if they had experience with FeNO testing because of its similarity to other types of asthma testing, but they reported valuing the potential of FeNO testing to provide more information about their condition as well as aid in the diagnosis and management of asthma.
Copyright © King's Printer for Ontario, 2024.
Figures


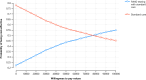
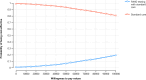



References
-
- Ontario Lung Association. Asthma diagnosis and management algorithm for primary care [Internet]. Toronto (ON): Lung Health Foundation; 2015. Available from: https://hcp.lunghealth.ca/wp-content/uploads/2020/02/Asthma-Diagnosis-an...
-
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012; 42(5): 650–8. - PubMed
-
- Seys SF, Long MB. The quest for biomarkers in asthma: challenging the T2 versus non-T2 paradigm. Eur Respir J. 2022; 59(2). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
