The role of ubiquitination in health and disease
- PMID: 39329019
- PMCID: PMC11424685
- DOI: 10.1002/mco2.736
The role of ubiquitination in health and disease
Abstract
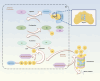
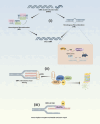
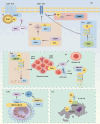
Ubiquitination is an enzymatic process characterized by the covalent attachment of ubiquitin to target proteins, thereby modulating their degradation, transportation, and signal transduction. By precisely regulating protein quality and quantity, ubiquitination is essential for maintaining protein homeostasis, DNA repair, cell cycle regulation, and immune responses. Nevertheless, the diversity of ubiquitin enzymes and their extensive involvement in numerous biological processes contribute to the complexity and variety of diseases resulting from their dysregulation. The ubiquitination process relies on a sophisticated enzymatic system, ubiquitin domains, and ubiquitin receptors, which collectively impart versatility to the ubiquitination pathway. The widespread presence of ubiquitin highlights its potential to induce pathological conditions. Ubiquitinated proteins are predominantly degraded through the proteasomal system, which also plays a key role in regulating protein localization and transport, as well as involvement in inflammatory pathways. This review systematically delineates the roles of ubiquitination in maintaining protein homeostasis, DNA repair, genomic stability, cell cycle regulation, cellular proliferation, and immune and inflammatory responses. Furthermore, the mechanisms by which ubiquitination is implicated in various pathologies, alongside current modulators of ubiquitination are discussed. Enhancing our comprehension of ubiquitination aims to provide novel insights into diseases involving ubiquitination and to propose innovative therapeutic strategies for clinical conditions.
Keywords: protein degradation; protein homeostasis; ubiquitin; ubiquitination.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Ubiquitination and deubiquitination: Implications on cancer therapy.Biochim Biophys Acta Gene Regul Mech. 2023 Dec;1866(4):194979. doi: 10.1016/j.bbagrm.2023.194979. Epub 2023 Aug 24. Biochim Biophys Acta Gene Regul Mech. 2023. PMID: 37633647 Review.
-
The Ubiquitin System and A20: Implications in Health and Disease.J Dent Res. 2021 Jan;100(1):10-20. doi: 10.1177/0022034520949486. Epub 2020 Aug 27. J Dent Res. 2021. PMID: 32853526 Free PMC article. Review.
-
The role of ubiquitin-binding domains in human pathophysiology.Crit Rev Clin Lab Sci. 2014 Oct;51(5):280-90. doi: 10.3109/10408363.2014.915287. Epub 2014 Jun 5. Crit Rev Clin Lab Sci. 2014. PMID: 24901807 Review.
-
Inhibition of proteasome reveals basal mitochondrial ubiquitination.J Proteomics. 2020 Oct 30;229:103949. doi: 10.1016/j.jprot.2020.103949. Epub 2020 Aug 31. J Proteomics. 2020. PMID: 32882436
-
Characterization of ubiquitination dependent dynamics in growth factor receptor signaling by quantitative proteomics.Mol Biosyst. 2011 Dec;7(12):3223-33. doi: 10.1039/c1mb05185g. Epub 2011 Sep 28. Mol Biosyst. 2011. PMID: 21956701
Cited by
-
The Role of the Ubiquitin System in Eye Diseases.Life (Basel). 2025 Mar 20;15(3):504. doi: 10.3390/life15030504. Life (Basel). 2025. PMID: 40141848 Free PMC article. Review.
-
Endothelial USP11 drives VEGFR2 signaling and angiogenesis via PRDX2/c-MYC axis.Angiogenesis. 2025 Apr 8;28(2):23. doi: 10.1007/s10456-025-09976-6. Angiogenesis. 2025. PMID: 40199774
-
USP7 promotes endothelial activation to aggravate sepsis-induced acute lung injury through PDK1/AKT/NF-κB signaling pathway.Cell Death Discov. 2025 Apr 17;11(1):183. doi: 10.1038/s41420-025-02481-1. Cell Death Discov. 2025. PMID: 40246841 Free PMC article.
-
The Emerging Role and Mechanism of E2/E3 Hybrid Enzyme UBE2O in Human Diseases.Biomedicines. 2025 Apr 29;13(5):1082. doi: 10.3390/biomedicines13051082. Biomedicines. 2025. PMID: 40426910 Free PMC article. Review.
-
N4BP3 Activates TLR4-NF-κB Pathway in Inflammatory Bowel Disease by Promoting K48-Linked IκBα Ubiquitination.J Inflamm Res. 2025 Jun 3;18:7167-7181. doi: 10.2147/JIR.S518155. eCollection 2025. J Inflamm Res. 2025. PMID: 40487287 Free PMC article.
References
-
- Wilkinson KD. Ubiquitin: a nobel protein. Cell. 2004;119(6):741‐745. - PubMed
-
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79‐87. - PubMed
-
- Ikeda F, Crosetto N, Dikic I. What determines the specificity and outcomes of ubiquitin signaling? Cell. 2010;143(5):677‐681. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
