Influence of Alzheimer's disease related neuropathology on local microenvironment gene expression in the human inferior temporal cortex
- PMID: 39329069
- PMCID: PMC11426291
- DOI: 10.1089/genbio.2023.0019
Influence of Alzheimer's disease related neuropathology on local microenvironment gene expression in the human inferior temporal cortex
Abstract
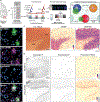
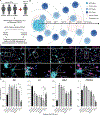
Neuropathological lesions in the brains of individuals affected with neurodegenerative disorders are hypothesized to trigger molecular and cellular processes that disturb homeostasis of local microenvironments. Here, we applied the 10x Genomics Visium Spatial Proteogenomics (Visium-SPG) platform, which couples spatial gene expression with immunofluorescence protein co-detection, to evaluate its ability to quantify changes in spatial gene expression with respect to amyloid-β (Aβ) and hyperphosphorylated tau (pTau) pathology in post-mortem human brain tissue from individuals with Alzheimer's disease (AD). We identified transcriptomic signatures associated with proximity to Aβ in the human inferior temporal cortex (ITC) during late-stage AD, which we further investigated at cellular resolution with combined immunofluorescence and single molecule fluorescent in situ hybridization (smFISH). The study provides a data analysis workflow for Visium-SPG, and the data represent a proof-of-principal for the power of multi-omic profiling in identifying changes in molecular dynamics that are spatially-associated with pathology in the human brain. We provide the scientific community with web-based, interactive resources to access the datasets of the spatially resolved AD-related transcriptomes at https://research.libd.org/Visium_SPG_AD/.
Keywords: Alzheimer's disease; RNA-protein co-detection; Spatially-resolved transcriptomics; amyloid-β (Aβ); neuropathology; phosphorylated tau (pTau).
Conflict of interest statement
Author disclosure statement SRW and MM are employees of 10x Genomics. All other authors declare no conflicts of interest.
Figures

Similar articles
-
Amyloid-beta and phosphorylated tau in post-mortem Alzheimer's disease retinas.Acta Neuropathol Commun. 2018 Dec 28;6(1):147. doi: 10.1186/s40478-018-0650-x. Acta Neuropathol Commun. 2018. PMID: 30593285 Free PMC article.
-
Spatially resolved transcriptomics reveals genes associated with the vulnerability of middle temporal gyrus in Alzheimer's disease.Acta Neuropathol Commun. 2022 Dec 21;10(1):188. doi: 10.1186/s40478-022-01494-6. Acta Neuropathol Commun. 2022. PMID: 36544231 Free PMC article.
-
VistoSeg: Processing utilities for high-resolution images for spatially resolved transcriptomics data.Biol Imaging. 2023 Nov 13;3:e23. doi: 10.1017/S2633903X23000235. eCollection 2023. Biol Imaging. 2023. PMID: 38510173 Free PMC article.
-
Neuropathology of Alzheimer's Disease.Neurotherapeutics. 2022 Jan;19(1):173-185. doi: 10.1007/s13311-021-01146-y. Epub 2021 Nov 2. Neurotherapeutics. 2022. PMID: 34729690 Free PMC article. Review.
-
Cognitive Decline in Preclinical Alzheimer's Disease: Amyloid-Beta versus Tauopathy.J Alzheimers Dis. 2018;61(1):265-281. doi: 10.3233/JAD-170490. J Alzheimers Dis. 2018. PMID: 29154274 Free PMC article. Review.
Cited by
-
Spatial Multi-Omics in Alzheimer's Disease: A Multi-Dimensional Approach to Understanding Pathology and Progression.Curr Issues Mol Biol. 2024 May 20;46(5):4968-4990. doi: 10.3390/cimb46050298. Curr Issues Mol Biol. 2024. PMID: 38785566 Free PMC article. Review.
-
SpotSweeper: spatially-aware quality control for spatial transcriptomics.bioRxiv [Preprint]. 2024 Jun 9:2024.06.06.597765. doi: 10.1101/2024.06.06.597765. bioRxiv. 2024. Update in: Nat Methods. 2025 Jul;22(7):1520-1530. doi: 10.1038/s41592-025-02713-3. PMID: 38895212 Free PMC article. Updated. Preprint.
-
Spatial domain detection using contrastive self-supervised learning for spatial multi-omics technologies.bioRxiv [Preprint]. 2024 Feb 4:2024.02.02.578662. doi: 10.1101/2024.02.02.578662. bioRxiv. 2024. Update in: Genome Res. 2025 Jul 1;35(7):1621-1632. doi: 10.1101/gr.279380.124. PMID: 38352580 Free PMC article. Updated. Preprint.
-
Spatial domain detection using contrastive self-supervised learning for spatial multi-omics technologies.Genome Res. 2025 Jul 1;35(7):1621-1632. doi: 10.1101/gr.279380.124. Genome Res. 2025. PMID: 40393810 Free PMC article.
-
Expanding our understanding of (mal)adapted stress physiology in psychiatric disorders: achieving single-cell characterisation of steroids and neuropeptides.Neurobiol Stress. 2025 Jun 6;37:100739. doi: 10.1016/j.ynstr.2025.100739. eCollection 2025 Jul. Neurobiol Stress. 2025. PMID: 40546293 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
