Safety assessment of sulfasalazine: a pharmacovigilance study based on FAERS database
- PMID: 39329122
- PMCID: PMC11424536
- DOI: 10.3389/fphar.2024.1452300
Safety assessment of sulfasalazine: a pharmacovigilance study based on FAERS database
Abstract
Background: Sulfasalazine is a widely used anti-inflammatory medication for treating autoimmune disorders such as ulcerative colitis (UC), Crohn's disease, and rheumatoid arthritis. However, its safety profile has not been systematically evaluated in real-world settings. By analyzing the FDA Adverse Event Reporting System (FAERS) database, we identified risk signals associated with adverse reactions to sulfasalazine, offering valuable insights for clinical decision-making and risk management.
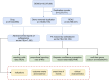
Methods: Reports of adverse events (AEs) associated with sulfasalazine, covering the period from Q1 2004 to Q4 2023, were extracted from the FAERS database. Detailed case information was aggregated to assess demographic characteristics. The associations between sulfasalazine and adverse events were evaluated using the Proportional Reporting Ratio (PRR), Reporting Odds Ratio (ROR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayes Geometric Mean (EBGM).
Results: We extracted 7,156 adverse event reports from the FAERS database where sulfasalazine was identified as the "Primary Suspect (PS)" drug. Using disproportionality analysis, we identified 101 preferred terms (PT) related to sulfasalazine across 24 organ systems. Notable adverse reactions consistent with the drug's labeling were observed, including Stevens-Johnson syndrome, agranulocytosis, eosinophilic pneumonia, and crystalluria. Additionally, novel positive signals not previously documented in the drug label were identified, including acute febrile neutrophilic dermatosis, aseptic meningitis, glomerulonephritis, and hepatosplenic T-cell lymphoma.
Conclusion: Most of the adverse reaction findings in this study are consistent with previous clinical research, and we have also identified new potential AEs associated with sulfasalazine. These findings provide valuable insights for the safety monitoring and clinical application of sulfasalazine.
Keywords: FDA adverse events reporting system; adverse drug reaction; adverse event; pharmacovigilance; sulfasalazine.
Copyright © 2024 Ye, Ding, Li, Tian, Wang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A disproportionality analysis of hydrocortisone-related adverse events: a real-world pharmacovigilance study using the FAERS database.Expert Opin Drug Saf. 2025 Apr 7:1-10. doi: 10.1080/14740338.2025.2487159. Online ahead of print. Expert Opin Drug Saf. 2025. PMID: 40163037
-
Drug-induced autoimmune-like hepatitis: A disproportionality analysis based on the FAERS database.PLoS One. 2025 Feb 6;20(2):e0317680. doi: 10.1371/journal.pone.0317680. eCollection 2025. PLoS One. 2025. PMID: 39913410 Free PMC article.
-
A real-world pharmacovigilance study and pharmacological analysis of sulfasalazine based on the FDA adverse event reporting system (FAERS) database.Expert Opin Drug Saf. 2025 May 5:1-9. doi: 10.1080/14740338.2025.2488241. Online ahead of print. Expert Opin Drug Saf. 2025. PMID: 40222949
-
Safety assessment of deutetrabenazine: real-world adverse event analysis from the FAERS database.Front Pharmacol. 2024 Dec 23;15:1498215. doi: 10.3389/fphar.2024.1498215. eCollection 2024. Front Pharmacol. 2024. PMID: 39764469 Free PMC article.
-
Suspected adverse drug reactions of rivaroxaban reported in the United States food and drug administration adverse event reporting system database: a pharmacovigilance study.Front Pharmacol. 2024 Sep 6;15:1399172. doi: 10.3389/fphar.2024.1399172. eCollection 2024. Front Pharmacol. 2024. PMID: 39309013 Free PMC article.
Cited by
-
Inactivated Cells and Metabolites of Saccharomyces boulardii Alleviate Inflammation Damage in Caco-2 Monolayer Cells and Mice with Ulcerative Colitis.Antioxidants (Basel). 2025 Jun 16;14(6):737. doi: 10.3390/antiox14060737. Antioxidants (Basel). 2025. PMID: 40563369 Free PMC article.
-
An integrated machine learning framework for developing and validating diagnostic models and drug predictions based on ulcerative colitis genes.Front Med (Lausanne). 2025 Jun 13;12:1571529. doi: 10.3389/fmed.2025.1571529. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40584713 Free PMC article.
-
Literature review of the clinical features of sulfasalazine-induced drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).Front Pharmacol. 2024 Dec 2;15:1488483. doi: 10.3389/fphar.2024.1488483. eCollection 2024. Front Pharmacol. 2024. PMID: 39687296 Free PMC article.
-
Development and Evaluation of Hydrogel-Based Sulfasalazine-Loaded Nanosponges for Enhanced Topical Psoriasis Therapy.Pharmaceuticals (Basel). 2025 Mar 10;18(3):391. doi: 10.3390/ph18030391. Pharmaceuticals (Basel). 2025. PMID: 40143167 Free PMC article.
References
LinkOut - more resources
Full Text Sources

