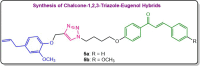
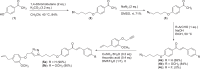
Step-by-step synthetic route to access eugenol-1,2,3-triazole-chalcone hybrid
- PMID: 39329152
- PMCID: PMC11426147
- DOI: 10.1016/j.mex.2024.102956
Step-by-step synthetic route to access eugenol-1,2,3-triazole-chalcone hybrid
Abstract
Molecular hybridization represents a strategic approach in drug design, where two or more pharmacophoric elements from distinct bioactive molecules are integrated into a single hybrid compound. In this study, we synthesized hybrid compounds of chalcone, triazole, and eugenol through straightforward reactions using 4-hydroxyacetophenone as the starting material. Initially, 4-hydroxyacetophenone (1) underwent alkylation with 1,4-dibromobutane to produce compound 2 with an 84 % yield. Compound 2 was then subjected to azidation, resulting in azidobutoxyacetophenone 3 with a 71 % yield. Subsequently, compound 3 was reacted with either benzaldehyde or 4-methoxybenzaldehyde via base-catalyzed aldol condensation, yielding azidobutoxychalcones 4a (69 %) and 4b (84 %). Finally, azide-alkyne [3+2] cycloaddition between 4a/4b and propargylated eugenol afforded chalcone derivatives bearing eugenol-1,2,3-triazole hybrids 5a and 5b, each with a 90 % yield.•Synthesized chalcones featuring an eugenol-1,2,3-triazole scaffold using 4-hydroxyacetophenone as the starting material.•Synthesis was accomplished through a four-step reaction sequence.•Products were obtained in good yield.
Keywords: 1,2,3-Triazole; 4-Hydroxyacetophenone; Chalcone; Eugenol; Molecular hybrid; Synthesis of chalcone containing an eugenol-1,2,3-triazole scaffold by aldol condensation and cycloaddition reactions.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A convenient method for the construction of triazole-bonded chalcone derivatives from acetophenone: Synthesis and free radical scavenging investigation.MethodsX. 2023 Aug 7;11:102322. doi: 10.1016/j.mex.2023.102322. eCollection 2023 Dec. MethodsX. 2023. PMID: 37608958 Free PMC article.
-
Design and Synthesis of Eugenol Derivatives Bearing a 1,2,3-Triazole Moiety for Papaya Protection against Colletotrichum gloeosporioides.J Agric Food Chem. 2024 Jun 5;72(22):12459-12468. doi: 10.1021/acs.jafc.4c00440. Epub 2024 May 21. J Agric Food Chem. 2024. PMID: 38771934 Free PMC article.
-
Synthesis, α-Glucosidase Inhibitory Activity and Molecular Docking Study of Chalcone Derivatives Bearing a 1H-1,2,3-Triazole Unit.Chem Pharm Bull (Tokyo). 2023;71(5):342-348. doi: 10.1248/cpb.c22-00844. Chem Pharm Bull (Tokyo). 2023. PMID: 37121684
-
Recent Advances in Bioactive Flavonoid Hybrids Linked by 1,2,3-Triazole Ring Obtained by Click Chemistry.Molecules. 2021 Dec 30;27(1):230. doi: 10.3390/molecules27010230. Molecules. 2021. PMID: 35011463 Free PMC article. Review.
-
Novel Hybrid Molecules Based on triazole-β-lactam as Potential Biological Agents.Mini Rev Med Chem. 2021;21(5):536-553. doi: 10.2174/1389557520666201027160436. Mini Rev Med Chem. 2021. PMID: 33109046 Review.
References
-
- Reddy P.N., Sharon N., Padmaja P., Ugale V.G., Lokwani D., Jain S., Pragati P., Anjali K. Molecular hybridization-based design, PASE three-component synthesis, antiproliferative activity and molecular modelling studies of N,N-dimethylaminophenyl substituted 5H-chromeno[2,3b]pyridine analogs. J. Mol. Struct. 2023;1286
-
- Khalil A.A., ur Rahman U., Khan M.R., Sahar A., Mehmood T., Khan M. Essential oil eugenol: sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017;7:32669–32681.
-
- Taleuzzaman M., Jain P., Verma R., Iqbal Z., Mirza M.A. Eugenol as potential drug candidate: a review. Curr. Top. Med. Chem. 2021;21(20):1804–1815. - PubMed
-
- Ardiansah B., Farhan A., Firdaus A., Ariyani T., Nasution M.A.F., Fadlan A., Cahyana A.H., Prabandari E.E., Menéndez J.C. Eugenol derivatives containing 1,2,3-triazole-chalcone hybrids for shikimate kinase inhibition. J. Saudi Chem. Soc. 2024;28(2)
LinkOut - more resources
Full Text Sources