Dietary "Beigeing" Fat Contains More Phosphatidylserine and Enhances Mitochondrial Function while Counteracting Obesity
- PMID: 39329159
- PMCID: PMC11425158
- DOI: 10.34133/research.0492
Dietary "Beigeing" Fat Contains More Phosphatidylserine and Enhances Mitochondrial Function while Counteracting Obesity
Abstract
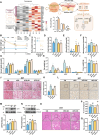
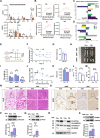
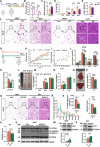
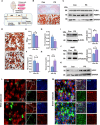
Activation of mitochondrial function and heat production in adipose tissue by the modification of dietary fat is a promising strategy against obesity. However, as an important source of lipids for ketogenic and daily diets, the function of fats extracted from different adipose tissue sites was largely unknown. In this study, we illustrated the function of fats extracted from adipose tissues with different "beigeing" properties in the ketogenic diet and identified lipid profiles of fats that facilitate energy expenditure. We found that the anti-obesity effect of ketogenic diets was potentiated by using "beigeing" fat [porcine subcutaneous adipose tissue (SAT)] as a major energy-providing ingredient. Through lipidomic analyses, phosphatidylserine (PS) was identified as a functional lipid activating thermogenesis in adipose tissue. Moreover, in vivo studies showed that PS induces adipose tissue thermogenesis and alleviates diet-induced obesity in mice. In vitro studies showed that PS promotes UCP1 expression and lipolysis of adipocytes. Mechanistically, PS promoted mitochondrial function in adipocytes via the ADCY3-cAMP-PKA-PGC1α pathway. In addition, PS-PGC1a binding may affect the stability of the PGC1α protein, which further augments PS-induced thermogenesis. These results demonstrated the efficacy of dietary SAT fats in diminishing lipid accumulation and the underlying molecular mechanism of PS in enhancing UCP1 expression and mitochondrial function. Thus, our findings suggest that as dietary fat, "beigeing" fat provides more beneficial lipids that contribute to the improvement of mitochondrial function, including PS, which may become a novel, nonpharmacological therapy to increase energy expenditure and counteract obesity and its related diseases.
Copyright © 2024 Yanbing Zhou et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Yellow Tea Stimulates Thermogenesis in Mice through Heterogeneous Browning of Adipose Tissues.Mol Nutr Food Res. 2021 Jan;65(2):e2000864. doi: 10.1002/mnfr.202000864. Epub 2021 Jan 7. Mol Nutr Food Res. 2021. PMID: 33258303
-
Intermittent fasting promotes type 3 innate lymphoid cells secreting IL-22 contributing to the beigeing of white adipose tissue.Elife. 2024 Mar 27;12:RP91060. doi: 10.7554/eLife.91060. Elife. 2024. PMID: 38536726 Free PMC article.
-
Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity.FASEB J. 2012 Sep;26(9):3728-37. doi: 10.1096/fj.12-204958. Epub 2012 May 25. FASEB J. 2012. PMID: 22637534
-
Recruitment of Thermogenic Fat: Trigger of Fat Burning.Front Endocrinol (Lausanne). 2021 Jul 22;12:696505. doi: 10.3389/fendo.2021.696505. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34367068 Free PMC article. Review.
-
Activation of Brown Adipose Tissue and Promotion of White Adipose Tissue Browning by Plant-based Dietary Components in Rodents: A Systematic Review.Adv Nutr. 2021 Dec 1;12(6):2147-2156. doi: 10.1093/advances/nmab084. Adv Nutr. 2021. PMID: 34265040 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

